当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactive molecular dynamics study of the oxidation behavior of iron-based alloy in supercritical water
Materials and Corrosion ( IF 1.6 ) Pub Date : 2021-05-14 , DOI: 10.1002/maco.202112328 Hong Xu 1 , Jing Qi 2
Materials and Corrosion ( IF 1.6 ) Pub Date : 2021-05-14 , DOI: 10.1002/maco.202112328 Hong Xu 1 , Jing Qi 2
Affiliation
|
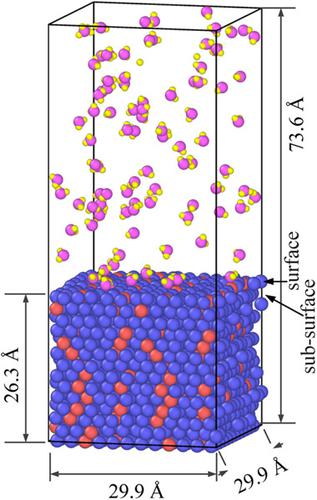
A theoretical study of the oxidation behavior of iron-based alloy in the supercritical water (SCW) has been carried out based on ReaxFF force-field molecular dynamics simulation. An atomic model has been proposed to simulate the initial chemisorption reactions and atoms diffusion behavior across the oxide layer. Simulation results imply that Cr addition has an important effect on the oxidation behavior of iron-based alloy. In the initial stage of oxidation, H2O prefers to adsorb on the Cr atom, and some species in the form of Cr(OH)4 are observed on the FeCr alloy surface. Once an initial oxide layer is formed, further oxidation is controlled by the migration of vacancy. The O vacancies are formed at the oxide/FeCr alloy interface and migrate toward the steam, whereas Fe vacancies are formed at the oxide/steam interface and migrate toward the FeCr alloy. Attributed to the stronger binding energy of O–Cr bond than O–Fe bond, the Cr diffusivity in the oxide is less than Fe atoms. Thus, double oxide layers, including the inner Fe–Cr–O layer and outer Fe–O layer, are formed on the FeCr alloy, which is in good agreement with previous experimental observation.
中文翻译:

铁基合金在超临界水中氧化行为的反应分子动力学研究
基于 ReaxFF 力场分子动力学模拟,对铁基合金在超临界水 (SCW) 中的氧化行为进行了理论研究。已经提出了一种原子模型来模拟初始化学吸附反应和跨氧化物层的原子扩散行为。模拟结果表明,Cr 添加对铁基合金的氧化行为有重要影响。在氧化的初始阶段,H 2 O 更喜欢吸附在 Cr 原子上,一些物质以 Cr(OH) 4的形式存在在 FeCr 合金表面观察到。一旦形成初始氧化层,进一步的氧化由空位的迁移控制。O空位在氧化物/FeCr合金界面处形成并向蒸汽迁移,而Fe空位在氧化物/蒸汽界面处形成并向FeCr合金迁移。由于 O-Cr 键的结合能比 O-Fe 键强,Cr 在氧化物中的扩散率小于 Fe 原子。因此,在 FeCr 合金上形成了双氧化层,包括内部 Fe-Cr-O 层和外部 Fe-O 层,这与先前的实验观察结果非常吻合。
更新日期:2021-05-14
中文翻译:

铁基合金在超临界水中氧化行为的反应分子动力学研究
基于 ReaxFF 力场分子动力学模拟,对铁基合金在超临界水 (SCW) 中的氧化行为进行了理论研究。已经提出了一种原子模型来模拟初始化学吸附反应和跨氧化物层的原子扩散行为。模拟结果表明,Cr 添加对铁基合金的氧化行为有重要影响。在氧化的初始阶段,H 2 O 更喜欢吸附在 Cr 原子上,一些物质以 Cr(OH) 4的形式存在在 FeCr 合金表面观察到。一旦形成初始氧化层,进一步的氧化由空位的迁移控制。O空位在氧化物/FeCr合金界面处形成并向蒸汽迁移,而Fe空位在氧化物/蒸汽界面处形成并向FeCr合金迁移。由于 O-Cr 键的结合能比 O-Fe 键强,Cr 在氧化物中的扩散率小于 Fe 原子。因此,在 FeCr 合金上形成了双氧化层,包括内部 Fe-Cr-O 层和外部 Fe-O 层,这与先前的实验观察结果非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号