Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simple amino silyl ether organocatalyst for asymmetric hetero Diels–Alder reaction of isatins with enones
Chirality ( IF 2.8 ) Pub Date : 2021-05-13 , DOI: 10.1002/chir.23319 Perumalsamy Parasuraman 1 , Divakar Ganesan 1 , Zubeda Begum 1 , Chigusa Seki 1 , Yuko Okuyama 2 , Eunsang Kwon 3 , Koji Uwai 1 , Michio Tokiwa 4 , Suguru Tokiwa 4 , Mitsuhiro Takeshita 4 , Hiroto Nakano 1
Chirality ( IF 2.8 ) Pub Date : 2021-05-13 , DOI: 10.1002/chir.23319 Perumalsamy Parasuraman 1 , Divakar Ganesan 1 , Zubeda Begum 1 , Chigusa Seki 1 , Yuko Okuyama 2 , Eunsang Kwon 3 , Koji Uwai 1 , Michio Tokiwa 4 , Suguru Tokiwa 4 , Mitsuhiro Takeshita 4 , Hiroto Nakano 1
Affiliation
|
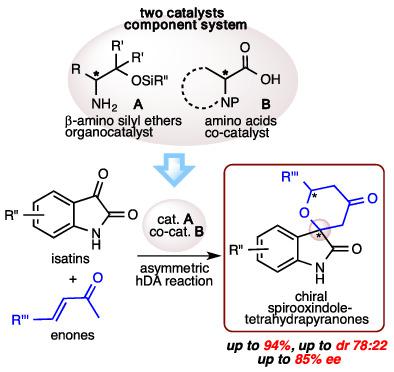
New two catalysts component system comprising of a primary β-amino silyl ethers as an organocatalyst and N-protected amino acids as a co-catalyst put together worked as an efficient organocatalyst system in the hetero Diels–Alder reaction of isatins with enones affording the chiral spirooxindole-tetrahydropyranones in good chemical yields and stereoselectivities (up to 94%, up to dr 78:22., up to 85% ee).
中文翻译:

用于靛红与烯酮的不对称杂 Diels-Alder 反应的简单氨基甲硅烷基醚有机催化剂
新的两种催化剂组分系统由作为有机催化剂的伯 β-氨基甲硅烷基醚和作为助催化剂的N-保护氨基酸组合在一起,在靛红与烯酮的杂 Diels-Alder 反应中作为有效的有机催化剂系统发挥作用,提供手性spirooxindole-tetrahydropyranones在良好的化学产率和立体选择性(高达94%,高达博士78:22。,高达85%的ee值)。
更新日期:2021-07-16
中文翻译:

用于靛红与烯酮的不对称杂 Diels-Alder 反应的简单氨基甲硅烷基醚有机催化剂
新的两种催化剂组分系统由作为有机催化剂的伯 β-氨基甲硅烷基醚和作为助催化剂的N-保护氨基酸组合在一起,在靛红与烯酮的杂 Diels-Alder 反应中作为有效的有机催化剂系统发挥作用,提供手性spirooxindole-tetrahydropyranones在良好的化学产率和立体选择性(高达94%,高达博士78:22。,高达85%的ee值)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号