当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering a stable complex of ERp44 with a designed peptide ligand for analyzing the mode of interaction of ERp44 with its clients
Peptide Science ( IF 1.5 ) Pub Date : 2021-05-14 , DOI: 10.1002/pep2.24230 Lutz Hampe 1 , Paul W. R. Harris 1, 2 , Ben Rushton 1, 3 , Mazdak Radjainia 1, 4 , Margaret A. Brimble 1, 2 , Alok K. Mitra 1
Peptide Science ( IF 1.5 ) Pub Date : 2021-05-14 , DOI: 10.1002/pep2.24230 Lutz Hampe 1 , Paul W. R. Harris 1, 2 , Ben Rushton 1, 3 , Mazdak Radjainia 1, 4 , Margaret A. Brimble 1, 2 , Alok K. Mitra 1
Affiliation
|
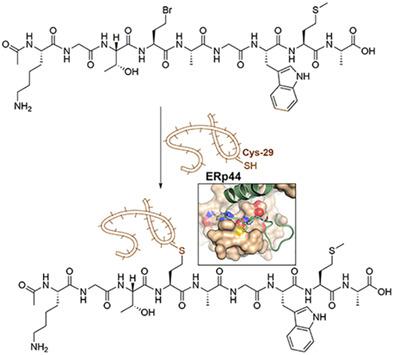
ERp44, a chaperone of the protein disulfide isomerase (PDI) family cycles between the endoplasmic reticulum (ER) and cis-Golgi compartments to act on a cohort of disparate proteins either to ensure their proper cellular localization or for the quality control of the correct assembly. This process involves intermolecular disulfide bond formation between the client protein and the conserved Cys29 in ERp44. We had identified a 9-amino acid peptide derived from an ERp44 client, adiponectin, as the motif interacting with ERp44 via a highly conserved cysteine (Cys39 of adiponectin). However, in order to reveal detailed insight into the mode of interaction, the generation of sizeable amounts of corresponding thiol-liganded ERp44 was unsuccessful under ambient conditions. Here we describe the production of a bromopeptide variant of this peptide ligand that led to large amounts of a pure, complex of peptide sufficient for structural studies in which Cys29 of Erp44 is covalently linked via a stable thioether bond. This method can be potentially used to rapidly probe putative, short, interacting peptide regions in other ERp44 clients to reveal their, hitherto unknown, mechanism of interaction with ERp44.
中文翻译:

用设计的肽配体设计稳定的 ERp44 复合物,用于分析 ERp44 与其客户的相互作用模式
ERp44,蛋白质二硫键异构酶 (PDI) 家族的分子伴侣在内质网 (ER)和顺式之间循环- 高尔基区室作用于一组不同的蛋白质,以确保它们正确的细胞定位或正确组装的质量控制。该过程涉及客户蛋白与 ERp44 中保守的 Cys29 之间的分子间二硫键形成。我们已经鉴定了一种源自 ERp44 客户脂联素的 9 氨基酸肽,作为通过高度保守的半胱氨酸(脂联素的 Cys39)与 ERp44 相互作用的基序。然而,为了揭示对相互作用模式的详细了解,在环境条件下生成大量相应的硫醇配体 ERp44 是不成功的。在这里,我们描述了该肽配体的溴肽变体的生产,该变体导致大量纯的、足以进行结构研究的肽复合物,其中 Erp44 的 Cys29 通过稳定的硫醚键共价连接。这种方法可潜在地用于快速探测其他 ERp44 客户中推定的、短的、相互作用的肽区域,以揭示其与 ERp44 相互作用的迄今为止未知的机制。
更新日期:2021-05-14
中文翻译:

用设计的肽配体设计稳定的 ERp44 复合物,用于分析 ERp44 与其客户的相互作用模式
ERp44,蛋白质二硫键异构酶 (PDI) 家族的分子伴侣在内质网 (ER)和顺式之间循环- 高尔基区室作用于一组不同的蛋白质,以确保它们正确的细胞定位或正确组装的质量控制。该过程涉及客户蛋白与 ERp44 中保守的 Cys29 之间的分子间二硫键形成。我们已经鉴定了一种源自 ERp44 客户脂联素的 9 氨基酸肽,作为通过高度保守的半胱氨酸(脂联素的 Cys39)与 ERp44 相互作用的基序。然而,为了揭示对相互作用模式的详细了解,在环境条件下生成大量相应的硫醇配体 ERp44 是不成功的。在这里,我们描述了该肽配体的溴肽变体的生产,该变体导致大量纯的、足以进行结构研究的肽复合物,其中 Erp44 的 Cys29 通过稳定的硫醚键共价连接。这种方法可潜在地用于快速探测其他 ERp44 客户中推定的、短的、相互作用的肽区域,以揭示其与 ERp44 相互作用的迄今为止未知的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号