当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic modelling of reactions for the synthesis of 2-methyl-5-ethyl pyridine
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-5-4 , DOI: 10.1039/d1re00085c Emanuele Moioli 1, 2, 3, 4, 5 , Leo Schmid 5, 6, 7, 8 , Peter Wasserscheid 1, 2, 3, 4 , Hannsjörg Freund 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-5-4 , DOI: 10.1039/d1re00085c Emanuele Moioli 1, 2, 3, 4, 5 , Leo Schmid 5, 6, 7, 8 , Peter Wasserscheid 1, 2, 3, 4 , Hannsjörg Freund 1, 2, 3, 4
Affiliation
|
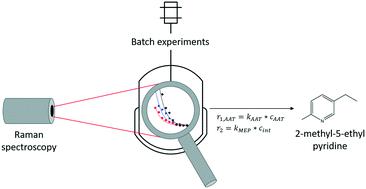
The kinetics of the acid catalyzed reactions of acetaldehyde ammonia trimer (AAT) and paraldehyde (para) to 2-methyl-5-ethyl pyridine (MEP) in the presence of an acid catalyst were investigated systematically. A reaction model has been developed based on experimental data. With full characterisation of side products it is possible to describe the reaction as a polymerisation and to understand the different reactivity of AAT and para, respectively. A kinetic model to describe the formation of MEP and the side products was developed using operando Raman spectroscopy. The model, involving four main reactions, is able to describe the formation of MEP and side products and incorporates the effects of reactant concentration, temperature and promoter concentration. This model is an important step that allows for further process intensification and evaluation of each of the two reaction routes.
中文翻译:

合成2-甲基-5-乙基吡啶的反应动力学模型
在酸催化剂的存在下,系统地研究了乙醛氨三聚体(AAT)和对甲醛(对位)生成2-甲基-5-乙基吡啶(MEP)的酸催化反应动力学。已经基于实验数据开发了反应模型。通过副产物的全面表征,可以将反应描述为聚合反应,并分别了解AAT和对位反应的不同性。描述MEP和副产物的形成动力学模型开发使用operando拉曼光谱。该模型涉及四个主要反应,能够描述MEP和副产物的形成,并结合了反应物浓度,温度和助催化剂浓度的影响。该模型是重要的步骤,可以进一步强化工艺流程并评估两条反应路线中的每条路线。
更新日期:2021-05-13
中文翻译:

合成2-甲基-5-乙基吡啶的反应动力学模型
在酸催化剂的存在下,系统地研究了乙醛氨三聚体(AAT)和对甲醛(对位)生成2-甲基-5-乙基吡啶(MEP)的酸催化反应动力学。已经基于实验数据开发了反应模型。通过副产物的全面表征,可以将反应描述为聚合反应,并分别了解AAT和对位反应的不同性。描述MEP和副产物的形成动力学模型开发使用operando拉曼光谱。该模型涉及四个主要反应,能够描述MEP和副产物的形成,并结合了反应物浓度,温度和助催化剂浓度的影响。该模型是重要的步骤,可以进一步强化工艺流程并评估两条反应路线中的每条路线。



























 京公网安备 11010802027423号
京公网安备 11010802027423号