Journal of Structural Biology ( IF 3.0 ) Pub Date : 2021-05-12 , DOI: 10.1016/j.jsb.2021.107741 G Baltulionis 1 , M Blight 2 , A Robin 2 , D Charalampopoulos 3 , K A Watson 4
|
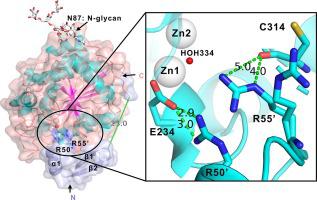
Leucyl aminopeptidase A from Aspergillus oryzae RIB40 (AO-LapA) is an exo-acting peptidase, widely utilised in food debittering applications. AO-LapA is secreted as a zymogen by the host and requires enzymatic cleavage of the autoinhibitory propeptide to reveal its full activity. Scarcity of structural data of zymogen aminopeptidases hampers a better understanding of the details of their molecular action of autoinhibition and how this might be utilised to improve the properties of such enzymes by recombinant methods for more effective bioprocessing. To address this gap in the literature, herein we report high-resolution crystal structures of recombinantly expressed AO-LapA precursor (AO-proLapA), mature LapA (AO-mLapA) and AO-mLapA complexed with reaction product l-leucine (AO-mLapA-Leu), all purified from Pichia pastoris culture supernatant. Our structures reveal a plausible molecular mechanism of LapA catalytic domain autoinhibition by propeptide and highlights the role of intramolecular chaperone (IMC). Our data suggest an absolute requirement for IMC in the maturation of cognate catalytic domain of AO-LapA. This observation is reinforced by our expression and refolding data of catalytic domain only (AO-refLapA) from Escherichia coli inclusion bodies, revealing a limited active conformation. Our work supports the notion that known synthetic aminopeptidase inhibitors and substrates mimic key polar contacts between propeptide and corresponding catalytic domain, demonstrated in our AO-proLapA zymogen crystal structure. Furthermore, understanding the atomic details of the autoinhibitory mechanism of cognate catalytic domains by native propeptides has wider reaching implications toward synthetic production of more effective inhibitors of bimetallic aminopeptidases and other dizinc enzymes that share an analogous reaction mechanism.
中文翻译:

前肽介导的自身抑制和分子间伴侣在亮氨酸氨基肽酶同源催化结构域成熟中的作用
来自米曲霉RIB40 的亮氨酰氨基肽酶 A (AO-LapA) 是一种外作用肽酶,广泛用于食品去苦味应用。AO-LapA 由宿主作为酶原分泌,需要酶促切割自抑制前肽以显示其全部活性。酶原氨肽酶结构数据的缺乏阻碍了对其自身抑制分子作用的细节的更好理解,以及如何通过重组方法改善此类酶的特性,从而实现更有效的生物加工。为了解决文献中的这一空白,本文报告了重组表达的 AO-LapA 前体 (AO -pro LapA)、成熟 LapA (AO- m LapA) 和 AO- m的高分辨率晶体结构。LapA 与反应产物 l-亮氨酸 (AO- m LapA-Leu) 复合,均从毕赤酵母培养上清液中纯化。我们的结构揭示了前肽对 LapA 催化结构域自身抑制的合理分子机制,并突出了分子内伴侣 (IMC) 的作用。我们的数据表明,在 AO-LapA 的同源催化结构域成熟中绝对需要 IMC 。我们的表达和仅来自大肠杆菌的催化结构域 (AO -ref LapA) 的重折叠数据强化了这一观察结果包涵体,显示有限的活性构象。我们的工作支持已知合成氨肽酶抑制剂和底物模拟前肽和相应催化结构域之间的关键极性接触的概念,这在我们的AO- pro LapA 酶原晶体结构中得到证明。此外,通过天然前肽了解同源催化结构域的自身抑制机制的原子细节,对于合成生产更有效的双金属氨肽酶抑制剂和其他具有类似反应机制的二锌酶抑制剂具有更广泛的意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号