Structure ( IF 5.7 ) Pub Date : 2021-05-10 , DOI: 10.1016/j.str.2021.04.009 Katharina Weinhäupl 1 , Yong Wang 2 , Audrey Hessel 1 , Martha Brennich 3 , Kresten Lindorff-Larsen 2 , Paul Schanda 1
|
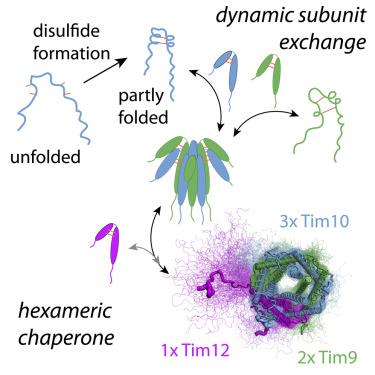
Tim chaperones transport membrane proteins to the two mitochondrial membranes. TIM9·10, a 70 kDa protein complex formed by 3 copies of Tim9 and Tim10, guides its clients across the aqueous compartment. The TIM9·10·12 complex is the anchor point at the inner-membrane insertase TIM22. The subunit composition of TIM9·10·12 remains debated. Joint NMR, small-angle X-ray scattering, and MD simulation data allow us to derive a structural model of the TIM9·10·12 assembly, with a 2:3:1 stoichiometry (Tim9:Tim10:Tim12). Both TIM9·10 and TIM9·10·12 hexamers are in a dynamic equilibrium with their constituent subunits, exchanging on a minutes timescale. NMR data establish that the subunits exhibit large conformational dynamics: when the conserved cysteines of the CX3C-Xn-CX3C motifs are formed, short α helices are formed, and these are fully stabilized only upon formation of the mature hexameric chaperone. We propose that the continuous subunit exchange allows mitochondria to control their level of inter-membrane space chaperones.
中文翻译:

基本线粒体伴侣复合物TIM9·10·12的结构和组装动力学
Tim 伴侣将膜蛋白转运至两个线粒体膜。TIM9·10 是由 3 个 Tim9 和 Tim10 拷贝形成的 70 kDa 蛋白质复合物,引导其客户穿过水室。TIM9·10·12 复合物是内膜插入酶 TIM22 的锚点。TIM9·10·12 的亚基组成仍有争议。联合 NMR、小角 X 射线散射和 MD 模拟数据使我们能够推导出具有 2:3:1 化学计量比 (Tim9:Tim10:Tim12) 的 TIM9·10·12 组件的结构模型。TIM9·10 和 TIM9·10·12 六聚体与其组成的亚基处于动态平衡状态,在几分钟的时间尺度上交换。核磁共振数据表明亚基表现出很大的构象动力学:当 CX 3 C-X n -CX 3的保守半胱氨酸形成 C 基序,形成短 α 螺旋,并且这些仅在成熟的六聚体伴侣形成时才完全稳定。我们建议连续的亚基交换允许线粒体控制其膜间空间伴侣的水平。



























 京公网安备 11010802027423号
京公网安备 11010802027423号