Science of the Total Environment ( IF 8.2 ) Pub Date : 2021-05-10 , DOI: 10.1016/j.scitotenv.2021.147668 Shengwu Yuan , Mei Ma , Xiaoshan Zhu , Yingnan Han , Kaifeng Rao , Zijian Wang
|
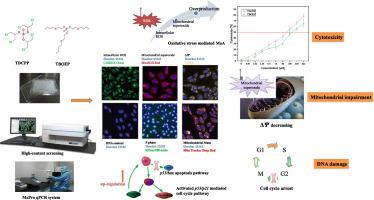
Tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and tris(2-butoxyethyl) phosphate (TBOEP), two of the most widely applied phosphorus-containing flame retardants (PFRs), have been shown to have adverse effects in different organisms, but their underlying toxicity mechanism remains to be elucidated. In the present study, the toxicological effects of TDCPP and TBOEP on human lung carcinoma (A549) cells and their associated mechanisms were investigated at the cellular, organelle and transcriptional levels. Cell Counting Kit-8 assay results showed that TDCPP and TBOEP reduced cell viability in a concentration-dependent manner. High-content screening assays showed that both PFRs caused mitochondrial impairment at the organelle level, decreasing the mitochondrial membrane potential and increasing the mitochondrial mass, and leading to increased cell cycle arrest-associated DNA damage that decreased the ratio of cells in S phase and increased the DNA content. In addition, oxidative stress was identified as a possible initial mechanistic process underlying PFR-induced cytotoxicity and mitochondrial impairment, as evidenced by intracellular reactive oxygen species and mitochondrial superoxide overproduction from environmentally relevant concentrations. Furthermore, p53 activation was shown to be involved in regulating DNA damage after A549 cell exposure to TDCPP and TBOEP. Quantitative PCR analyses showed that TDCPP and TBOEP triggered p53/p21-mediated cell cycle arrest pathways, indicating that these PFRs can activate p53, and promote associated functional repair. However, the p53/bax-mediated apoptosis pathway was not activated following exposure to PFRs at specific concentrations. Our results showed that TDCPP and TBOEP can cause cytotoxicity, mitochondrial impairment and cell cycle arrest-associated DNA damage in A549 cells, suggesting that oxidative stress is the initial molecular event by which these adverse outcomes are induced.
中文翻译:

磷酸三(1,3-二氯-2-丙基)和磷酸三(2-丁氧基乙基)酯诱导的A549细胞的细胞毒性,线粒体损伤,DNA损伤及相关机制
磷酸三(1,3-二氯-2-丙基)酯(TDCPP)和磷酸三(2-丁氧基乙基)酯(TBOEP)已显示出不利的影响在不同的生物中,它们的潜在毒性机制仍有待阐明。在本研究中,从细胞,细胞器和转录水平研究了TDCPP和TBOEP对人肺癌(A549)细胞的毒理作用及其相关机制。Cell Counting Kit-8分析结果表明TDCPP和TBOEP以浓度依赖性方式降低了细胞活力。高含量筛选试验表明,两种PFR都会在细胞器水平上引起线粒体损伤,从而降低线粒体膜电位并增加线粒体质量,并导致细胞周期停滞相关的DNA损伤增加,从而降低S期细胞比例并增加DNA含量。此外,氧化应激被认为是PFR诱导的细胞毒性和线粒体损伤的潜在可能的初始机制,这一点可以通过细胞内活性氧和环境相关浓度的线粒体超氧化物的过量生产来证明。此外,p53激活被证明与A549细胞暴露于TDCPP和TBOEP后调节DNA损伤有关。定量PCR分析表明TDCPP和TBOEP触发了 氧化应激被认为是PFR诱导的细胞毒性和线粒体损伤的潜在可能的初始机制,这一点可以通过细胞内活性氧和环境相关浓度的线粒体超氧化物的过量生产来证明。此外,p53激活被证明与A549细胞暴露于TDCPP和TBOEP后调节DNA损伤有关。定量PCR分析表明TDCPP和TBOEP触发了 氧化应激被认为是PFR诱导的细胞毒性和线粒体损伤的潜在可能的初始机制,这一点可以通过细胞内活性氧和环境相关浓度的线粒体超氧化物的过量生产来证明。此外,p53激活被证明与A549细胞暴露于TDCPP和TBOEP后调节DNA损伤有关。定量PCR分析表明TDCPP和TBOEP触发了p53 / p21介导的细胞周期阻滞途径,表明这些PFR可以激活p53,并促进相关的功能修复。但是,p53 / bax介导的细胞凋亡途径在暴露于特定浓度的PFR后未激活。我们的结果表明,TDCPP和TBOEP可以在A549细胞中引起细胞毒性,线粒体损伤和细胞周期停滞相关的DNA损伤,这表明氧化应激是诱导这些不良后果的最初分子事件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号