Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-05-09 , DOI: 10.1016/j.jhazmat.2021.126052 Yu-Hua Wu 1 , Yu-Long Ma 1 , Yong-Gang Sun 1 , Wen-Xin Ji 1 , Feng Lin 1 , Yi-Peng Yang 1 , Li-Juan Ma 1 , Chun-Hong Zhu 1 , Yi-Jie Xu 1 , Qing Miao 1
|
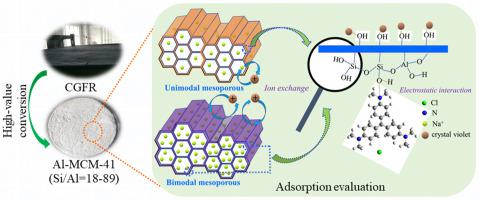
The development of synthetic methods to obtain high value-added mesoporous Al-MCM-41 from a low-cost silicon-aluminum source with low toxicity is an active research topic in solid waste resource utilization. In particular, the controlled synthesis of MCM-41 with a two-level pore distribution is a challenging task. In this work, the synthesis of unimodal and bimodal mesoporous Al-MCM-41s was achieved using acids with different degrees of ionization from coal gasification fine residue (CGFR) as bulk solid waste generated by the coal gasification process. We determined that the degree of acid ionization affected the self-assembly of inorganic/organic species as well as condensation processes, resulting in some changes of the hexagonal mesoscopic structure. The unimodal mesoporous Al-MCM-41 with acetic acid HAc and bimodal mesoporous Al-MCM-41s with an inorganic acid environment (HCl, HNO3 or H2SO4) could be effectively prepared in a controllable manner by the silicon and aluminum source obtained at alkali dissolution time 6 h and crystallization conditions at pH 10.5 and 383 K in 72 h. Moreover, the synthesis of Al-MCM-41-HAc with different SiO2/Al2O3 molar ratios (18–89) could also be realized by different alkali dissolution times. And alkali dissolution time (2–24 h) and the crystallization conditions (pH 4.5–11.5, temperatures 373–393 K, and time 48–96 h) also affected the formation of unimodal and bimodal mesoporous Al-MCM-41-HAc. In addition, the maximum adsorption amount onto bimodal mesoporous Al-MCM-41-H2SO4 (476.19 mg g−1 at 308 K) was larger than that onto unimodal mesoporous Al-MCM-41-HAc (243.90 mg g−1 at 303 K). The mesoporous Al-MCM-41s showed good stability.
中文翻译:

酸离子化对煤气化细渣中双峰介孔Al-MCM-41s形成机理的影响及吸附性能的评价
从低成本,低毒性的硅铝源中获得高附加值的介孔Al-MCM-41的合成方法的研究是固体废物资源利用的积极研究课题。特别是,具有两级孔分布的MCM-41的受控合成是一项艰巨的任务。在这项工作中,单峰和双峰介孔Al-MCM-41s的合成是利用煤气化细残渣(CGFR)作为煤气化过程中产生的大块固体废物,采用不同电离度的酸实现的。我们确定酸离子化的程度会影响无机/有机物的自组装以及缩合过程,从而导致六边形介观结构发生某些变化。通过碱溶解时间6 h和pH 10.5和383 K下72 h的结晶条件下获得的硅和铝源,可以可控地有效地制备3或H 2 SO 4)。此外,还可以通过不同的碱溶解时间来合成具有不同SiO 2 / Al 2 O 3摩尔比(18-89)的Al-MCM-41-HAc 。碱溶解时间(2-24小时)和结晶条件(pH 4.5-11.5,温度373-393 K,时间48-96小时)也影响了单峰和双峰介孔Al-MCM-41-HAc的形成。另外,双峰介孔Al-MCM-41-H 2 SO 4的最大吸附量(476.19毫克克-1在308 K)比大到单峰孔的Al-MCM-41-的HAc(243.90毫克克-1在303 K)。中孔Al-MCM-41s显示出良好的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号