Sustainable Chemistry and Pharmacy ( IF 5.5 ) Pub Date : 2021-05-08 , DOI: 10.1016/j.scp.2021.100437 Farshid Ghorbani , Soran Kamari , Fatemeh Askari , Hadieh Molavi , Somayeh Fathi
|
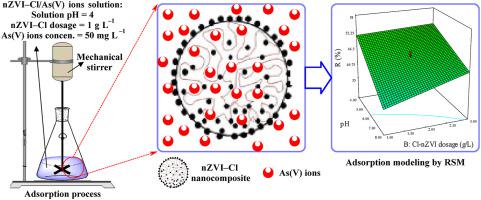
By combination of the nano zero–valent iron (nZVI) nanoparticles and clinoptilolite (Cl) natural zeolite and by chemical reduction method, the nZVI–Cl nanocomposite was produced. The physical and chemical characteristics of the produced nanocomposite were examined using FE–SEM, EDX, VSM and zeta potential analyses and its fine structure was confirmed. The produced nanocomposite was applied for efficient As(V) heavy metal ions removal from aqueous media as a novel eco–friendly adsorbent. The adsorption process was optimized using a minimum number of designed experiments by Design–Expert software using central composite design (CCD) and response surface methodology (RSM) and the adsorption optimum conditions were determined as solution pH of 4, nZVI–Cl dosage of 1 g L−1 and As(V) concentration of 50 mg L−1 by the numerical optimization of the software. In these conditions, the removal efficiency was 88.10% and desirability parameter was 0.986. The adsorption kinetic study showed that the chemisorption effectively controls the adsorption process by a better fit of pseudo–second order model with the experimental data. The adsorption isotherms study demonstrated that the Langmuir model had the best fit with the experimental data, proving homogeneous surface of nZVI–Cl adsorbent and monolayer adsorption of As(V) ions on it. The adsorption thermodynamic study illustrated that the adsorption is thermodynamically spontaneous and exothermic in nature. Also, the adsorbent reusability test verified the adsorbent stability after five consecutive adsorption–desorption cycles without a tangible reduction in removal efficiency.
中文翻译:

生产nZVI-Cl纳米复合材料作为一种新型生态友好型吸附剂,可从水性介质中有效去除As(V)离子:通过响应面法进行吸附建模
通过将纳米零价铁(nZVI)纳米颗粒与斜发沸石(Cl)天然沸石结合并通过化学还原方法,制得了nZVI-Cl纳米复合材料。使用FE–SEM,EDX,VSM和zeta电位分析检查了所生产纳米复合材料的物理和化学特性,并确认了其精细结构。所生产的纳米复合材料可作为一种新型的生态友好型吸附剂,用于从水性介质中有效去除As(V)重金属离子。通过使用中央复合设计(CCD)和响应表面方法(RSM)的Design–Expert软件进行的最少设计实验,对吸附过程进行了优化,并确定了吸附的最佳条件,即溶液的pH为4,nZVI–Cl的添加量为1 g L -1和50 mg L的As(V)浓度-1通过软件的数值优化。在这些条件下,去除效率为88.10%,期望参数为0.986。吸附动力学研究表明,化学吸附可以通过伪二阶模型与实验数据更好地拟合来有效地控制吸附过程。吸附等温线研究表明,Langmuir模型与实验数据最吻合,证明nZVI-Cl吸附剂的表面均匀并且在其上单层吸附了As(V)离子。吸附热力学研究表明,吸附在本质上是热力学自发的并且是放热的。另外,吸附剂可重复使用性测试在连续五个吸附-解吸循环后证实了吸附剂的稳定性,而去除效率没有明显降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号