Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2021-05-08 , DOI: 10.1016/j.yjmcc.2021.05.002 Dorothee Jakob 1 , Alexander Klesen 1 , Benoit Allegrini 2 , Elisa Darkow 3 , Diana Aria 4 , Ramona Emig 5 , Ana Simon Chica 1 , Eva A Rog-Zielinska 1 , Tim Guth 1 , Friedhelm Beyersdorf 6 , Fabian A Kari 6 , Susanne Proksch 7 , Stéphane N Hatem 8 , Matthias Karck 9 , Stephan R Künzel 10 , Hélène Guizouarn 2 , Constanze Schmidt 11 , Peter Kohl 5 , Ursula Ravens 1 , Rémi Peyronnet 1
|
Aims
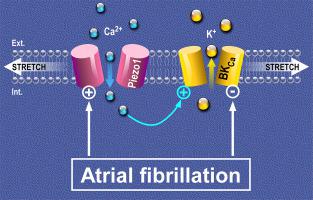
Atrial Fibrillation (AF) is an arrhythmia of increasing prevalence in the aging populations of developed countries. One of the important indicators of AF is sustained atrial dilatation, highlighting the importance of mechanical overload in the pathophysiology of AF. The mechanisms by which atrial cells, including fibroblasts, sense and react to changing mechanical forces, are not fully elucidated. Here, we characterise stretch-activated ion channels (SAC) in human atrial fibroblasts and changes in SAC- presence and activity associated with AF.
Methods and results
Using primary cultures of human atrial fibroblasts, isolated from patients in sinus rhythm or sustained AF, we combine electrophysiological, molecular and pharmacological tools to identify SAC. Two electrophysiological SAC- signatures were detected, indicative of cation-nonselective and potassium-selective channels. Using siRNA-mediated knockdown, we identified the cation-nonselective SAC as Piezo1. Biophysical properties of the potassium-selective channel, its sensitivity to calcium, paxilline or iberiotoxin (blockers), and NS11021 (activator), indicated presence of calcium-dependent ‘big potassium channels’ (BKCa). In cells from AF patients, Piezo1 activity and mRNA expression levels were higher than in cells from sinus rhythm patients, while BKCa activity (but not expression) was downregulated. Both Piezo1-knockdown and removal of extracellular calcium from the patch pipette resulted in a significant reduction of BKCa current during stretch. No co-immunoprecipitation of Piezo1 and BKCa was detected.
Conclusions
Human atrial fibroblasts contain at least two types of ion channels that are activated during stretch: Piezo1 and BKCa. While Piezo1 is directly stretch-activated, the increase in BKCa activity during mechanical stimulation appears to be mainly secondary to calcium influx via SAC such as Piezo1. During sustained AF, Piezo1 is increased, while BKCa activity is reduced, highlighting differential regulation of both channels. Our data support the presence and interplay of Piezo1 and BKCa in human atrial fibroblasts in the absence of physical links between the two channel proteins.
中文翻译:

人心房成纤维细胞中的 Piezo1 和 BKCa 通道:心房颤动中的相互作用和重塑
目标
心房颤动 (AF) 是一种心律失常,在发达国家的老龄人口中越来越普遍。房颤的重要指标之一是持续性心房扩张,突出了机械过载在房颤病理生理学中的重要性。心房细胞(包括成纤维细胞)感知变化的机械力并对其作出反应的机制尚未完全阐明。在这里,我们描述了人心房成纤维细胞中的拉伸激活离子通道 (SAC) 以及与 AF 相关的 SAC 存在和活动的变化。
方法和结果
我们使用从窦性心律或持续 AF 患者中分离出来的人心房成纤维细胞的原代培养物,结合电生理学、分子学和药理学工具来识别 SAC。检测到两种电生理 SAC 特征,表明阳离子非选择性和钾选择性通道。使用 siRNA 介导的敲低,我们将阳离子非选择性 SAC 鉴定为 Piezo1。钾选择性通道的生物物理特性,它对钙、桩碱或伊比利亚毒素(阻滞剂)和 NS11021(激活剂)的敏感性表明存在钙依赖性“大钾通道”(BK Ca)。在 AF 患者的细胞中,Piezo1 活性和 mRNA 表达水平高于窦性心律患者的细胞,而 BK Ca活性(但不是表达)被下调。Piezo1 敲低和从贴片移液器中去除细胞外钙导致拉伸期间 BK Ca电流显着降低。没有检测到 Piezo1 和 BK Ca的免疫共沉淀。
结论
人心房成纤维细胞包含至少两种在拉伸过程中被激活的离子通道:Piezo1 和BK Ca。虽然 Piezo1 是直接拉伸激活的,但机械刺激期间 BK Ca活性的增加似乎主要是继发于通过SAC(如 Piezo1)的钙流入。在持续 AF 期间,Piezo1 增加,而 BK Ca活性降低,突出了两个通道的差异调节。我们的数据支持在两个通道蛋白之间没有物理联系的情况下,人心房成纤维细胞中Piezo1 和 BK Ca的存在和相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号