Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-05-08 , DOI: 10.1016/j.jhazmat.2021.126016 Yu Kong 1 , Hongyu Sun 2 , Siyu Zhang 3 , Bing Zhao 3 , Qing Zhao 3 , Xuejiao Zhang 3 , Haibo Li 4
|
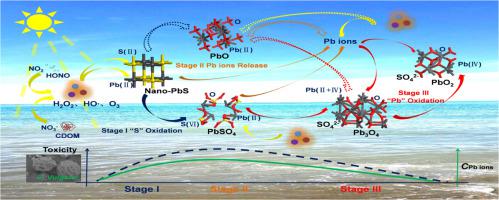
Lead sulfide nanoparticle (nano-PbS) released into environment can cause hazards to human or ecosystem. Nano-PbS potentially undergoes oxidation in the environment, but oxidation mechanism is not understood yet. Herein, oxidation kinetics and products of nano-PbS by ozone (O3), hydrogen peroxide (H2O2) and hydroxyl radical (HO·) in the atmosphere or natural water were investigated. Results show that oxidation process of nano-PbS can be divided into three stages, producing sulfate, ions and oxides of lead in sequence. O3 or HO·leads to faster release of ionic lead from nano-PbS in the initial stage than H2O2, but causes significant decrease of ionic lead by transforming divalent lead to tetravalent lead oxides in the second or third stage. Toxicity determined taking Chlorella Vulgaris as an example follows an order of PbO2 < Pb3O4 < nano-PbS < PbO < PbSO4. Toxicity of lead particles is mainly determined by sizes influencing cellular uptake and solubility product constant (Ksp) related with dissolution of lead in cells. The results indicate that the toxicity of nano-PbS increases in an initial oxidation stage and decreases in further oxidation stages. This study provides new insights into environmental behavior of nano-PbS and mechanism understandings for assessing ecological risks of nano-PbS.
中文翻译:

硫化铅纳米粒子在大气或天然水中的氧化过程及其对小球藻毒性的影响
释放到环境中的硫化铅纳米粒子(nano-PbS)可能对人类或生态系统造成危害。纳米PbS在环境中可能会发生氧化,但氧化机理尚不明确。本文研究了大气或天然水中臭氧(O 3),过氧化氢(H 2 O 2)和羟基自由基(HO·)的氧化动力学及其产物。结果表明,纳米PbS的氧化过程可分为三个阶段,依次产生硫酸根,离子和铅的氧化物。在初始阶段,O 3或HO·比H 2 O 2更快地从纳米PbS中释放出离子铅。,但是在第二阶段或第三阶段中,通过将二价铅转变为四价氧化铅,会导致离子铅的显着减少。以小球藻为例确定的毒性遵循PbO 2 <Pb 3 O 4 <纳米PbS <PbO <PbSO 4的顺序。铅颗粒的毒性主要由影响细胞摄取的大小和溶解度乘积常数(K sp)与铅在细胞中的溶解有关。结果表明,纳米PbS的毒性在初始氧化阶段增加而在进一步氧化阶段下降。这项研究为纳米PbS的环境行为提供了新见解,并为评估纳米PbS的生态风险提供了机理认识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号