Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2021-08-31 , DOI: 10.2174/0929866528666210316104919 Imran Mohsin 1 , Li-Qing Zhang 2 , Duo-Chuan Li 2 , Anastassios C Papageorgiou 1
|
Background: Thermophilic fungi have recently emerged as a promising source of thermostable enzymes. Superoxide dismutases are key antioxidant metalloenzymes with promising therapeutic effects in various diseases, both acute and chronic. However, structural heterogeneity and low thermostability limit their therapeutic efficacy.
Objective: Although several studies from hypethermophilic superoxide dismutases (SODs) have been reported, information about Cu,Zn-SODs from thermophilic fungi is scarce. Chaetomium thermophilum is a thermophilic fungus that could provide proteins with thermophilic properties.
Methods: The enzyme was expressed in Pichia pastoris cells and crystallized using the vapor-diffusion method. X-ray data were collected, and the structure was determined and refined to 1.56 Å resolution. Structural analysis and comparisons were carried out.
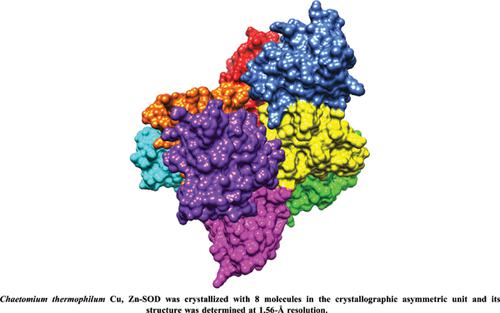
Results: The presence of 8 molecules (A through H) in the asymmetric unit resulted in four different interfaces. Molecules A and F form the typical homodimer which is also found in other Cu,Zn- SODs. Zinc was present in all subunits of the structure while copper was found in only four subunits with reduced occupancy (C, D, E and F).
Conclusion: The ability of the enzyme to form oligomers and the elevated Thr:Ser ratio may be contributing factors to its thermal stability. Two hydrophobic residues that participate in interface formation and are not present in other CuZn-SODs may play a role in the formation of new interfaces and the oligomerization process. The CtSOD crystal structure reported here is the first Cu,Zn-SOD structure from a thermophilic fungus.
中文翻译:

来自嗜热真菌 Chaetomium thermophilum 的 Cu,Zn 超氧化物歧化酶的晶体结构
背景:嗜热真菌最近成为热稳定酶的有前途的来源。超氧化物歧化酶是关键的抗氧化金属酶,在各种急性和慢性疾病中具有良好的治疗效果。然而,结构异质性和低热稳定性限制了它们的治疗效果。
目的:虽然已经报道了一些关于超嗜热超氧化物歧化酶 (SOD) 的研究,但关于来自嗜热真菌的 Cu、Zn-SOD 的信息很少。Chaetomium thermophilum 是一种嗜热真菌,可为蛋白质提供嗜热特性。
方法:该酶在毕赤酵母细胞中表达,并使用蒸气扩散法结晶。收集 X 射线数据,确定结构并将其细化至 1.56 Å 分辨率。进行了结构分析和比较。
结果:在不对称单元中存在 8 个分子(A 到 H)导致四个不同的界面。分子 A 和 F 形成典型的同型二聚体,在其他 Cu、Zn-SOD 中也发现了这种同型二聚体。锌存在于结构的所有亚基中,而铜仅存在于占有率降低的四个亚基(C、D、E 和 F)中。
结论:酶形成寡聚体的能力和升高的 Thr:Ser 比率可能是其热稳定性的促成因素。参与界面形成且在其他 CuZn-SOD 中不存在的两个疏水残基可能在新界面的形成和低聚过程中发挥作用。此处报道的 CtSOD 晶体结构是来自嗜热真菌的第一个 Cu、Zn-SOD 结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号