Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2021-08-31 , DOI: 10.2174/0929866528666210215140231 Rohit Kumar Singh 1 , Devbrat Kumar 1 , Samudrala Gourinath 1
|
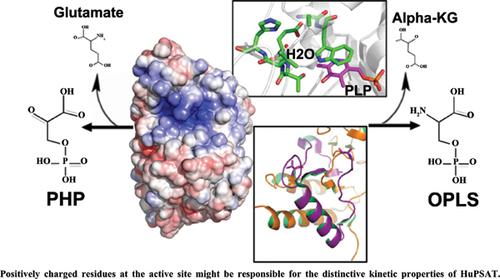
Serine is ubiquitously synthesized in all living organisms from the glycolysis intermediate 3-phosphoglycerate (PGA) by phosphoserine biosynthetic pathway, consisting of three different enzymes, namely: 3-phosphoglycerate dehydrogenase (PGDH), phosphoserine aminotransferase (PSAT), and phosphoserine phosphatase (PSP). Any functional defect or mutation in these enzymes may cause deliberating conditions, such as colon cancer progression and chemoresistance in humans. Phosphoserine aminotransferase (PSAT) is the second enzyme in this pathway that converts phosphohydroxypyruvate (PHP) to O-phospho-L-serine (OPLS).
Humans encode two isoforms of this enzyme: PSAT1 and PSAT2. PSAT1 exists as a functional dimer, where each protomer has a large and a small domain; each large domain contains a Lys residue that covalently binds PLP. The PLP-binding site of human PSAT1 and most of its active site residues are highly conserved in all known PSAT structures except for Cys-80. Interestingly, Two PSAT structures from different organisms show halide binding near their active site. While the human PSAT1 shows a water molecule at this site with different interacting residues, suggesting the inability of halide binding in the human enzyme. Analysis of the human PSAT1 structure showed a big patch of positive charge around the active site, in contrast to the bacterial PSATs. Compared to human PSAT1, the PSAT2 isoform lacks 46 residues at its C-terminal tail. This tail region is present at the opening of the active site as observed in the other PSAT structures. Further structural work on human PSAT2 may reveal the functional importance of these 46 residues.
中文翻译:

磷酸丝氨酸氨基转移酶保留了从微生物到高等真核生物的活性位点,并具有较小的偏差
丝氨酸在所有生物体中通过磷酸丝氨酸生物合成途径由糖酵解中间体 3-磷酸甘油酸 (PGA) 普遍合成,由三种不同的酶组成,即:3-磷酸甘油酸脱氢酶 (PGDH)、磷酸丝氨酸氨基转移酶 (PSAT) 和磷酸丝氨酸磷酸酶 (PSP) )。这些酶的任何功能缺陷或突变都可能导致故意的状况,例如人类的结肠癌进展和化学耐药性。磷酸丝氨酸氨基转移酶 (PSAT) 是该途径中的第二种酶,可将磷酸羟基丙酮酸 (PHP) 转化为 O-磷酸-L-丝氨酸 (OPLS)。
人类编码这种酶的两种同工型:PSAT1 和 PSAT2。PSAT1 以功能性二聚体形式存在,其中每个原体都有一个大域和一个小域;每个大域都包含一个 Lys 残基,可与 PLP 共价结合。人类 PSAT1 的 PLP 结合位点及其大部分活性位点残基在除 Cys-80 之外的所有已知 PSAT 结构中都高度保守。有趣的是,来自不同生物体的两个 PSAT 结构在其活性位点附近显示出卤化物结合。而人类 PSAT1 在该位点显示一个水分子,具有不同的相互作用残基,表明人类酶中无法结合卤化物。对人类 PSAT1 结构的分析显示,与细菌 PSATs 相比,活性位点周围有一大片正电荷。与人类 PSAT1 相比,PSAT2 同种型在其 C 端尾部缺少 46 个残基。如在其他 PSAT 结构中所观察到的,该尾部区域存在于活性位点的开口处。对人类 PSAT2 的进一步结构工作可能会揭示这 46 个残基的功能重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号