Applied Surface Science ( IF 6.3 ) Pub Date : 2021-05-07 , DOI: 10.1016/j.apsusc.2021.150017 Yuyue Gao , Yan Shen , Quan Zhu , Jianyi Ma , Haisheng Ren
|
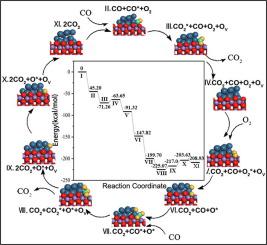
The interface formed by metal cluster supported on (reducible) transition metal oxides (TMOs) is usually responsible for the excellent activity of the redox reaction in the heterogeneously catalytic field. In this work, the catalytic combustion of CO was selected as a typical probe reaction to theoretically reveal the features of Pt13/β-MnO2 (1 1 0) catalyst, which possesses four active sites i.e., interface, β-MnO2 (1 1 0) facet, metallicity (Pt0) and partial oxidized Pt (Ptx+). All active sites are carefully investigated by the characteristics of CO oxidation. For Pt0 and Ptx+ sites, the adsorption energies of CO are larger than those of O2, which might block O2 active sites and cause CO poison. Meanwhile, CO oxidation by the dissociated O atom has the relatively high reaction energy barrier. Thus, Pt0 and Ptx+ are not good sites for CO oxidation. For β-MnO2 (1 1 0) facet, O2 dissociation is not easy with a high barrier for vacancies healing. In contrast, the most favorable mechanism of CO oxidation is the catalytic cycle at the interface, with low reaction energy barriers in the entire pathway. We hope this comprehensive work can provide a meaningful reference towards the design of heterogeneous catalysts.
中文翻译:

MnO 2负载的Pt 13团簇上CO氧化的机理和能量学
由负载在(可还原的)过渡金属氧化物(TMO)上的金属簇形成的界面通常负责在非均相催化领域中氧化还原反应的出色活性。在这项工作中,CO的催化燃烧被选为典型的探针反应,理论上揭示了Pt的特征13 /β-MnO的2(110)催化剂,其具有4个活性位点,即,接口,β-MnO的2( 1 1 0)刻面,金属性(Pt 0)和部分氧化的Pt(Pt x +)。通过CO氧化的特性仔细研究了所有活性位。对于Pt 0和Pt x +位,CO的吸附能大于O 2的吸附能,这可能会阻塞O 2活性位并引起CO中毒。同时,离解的O原子对CO的氧化具有较高的反应能垒。因此,Pt 0和Pt x +不是CO氧化的良好位点。β-MnO的2(110)面,O- 2离解是不容易的具有高阻挡空缺愈合。相反,CO氧化的最有利机制是界面处的催化循环,整个路径中的反应能垒较低。我们希望这项全面的工作可以为多相催化剂的设计提供有意义的参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号