当前位置:
X-MOL 学术
›
Am. J. Primatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer's-like neuropathology: An evolutionary perspective
American Journal of Primatology ( IF 2.4 ) Pub Date : 2021-05-07 , DOI: 10.1002/ajp.23254 Amy F T Arnsten 1 , Dibyadeep Datta 1 , Todd M Preuss 2
American Journal of Primatology ( IF 2.4 ) Pub Date : 2021-05-07 , DOI: 10.1002/ajp.23254 Amy F T Arnsten 1 , Dibyadeep Datta 1 , Todd M Preuss 2
Affiliation
|
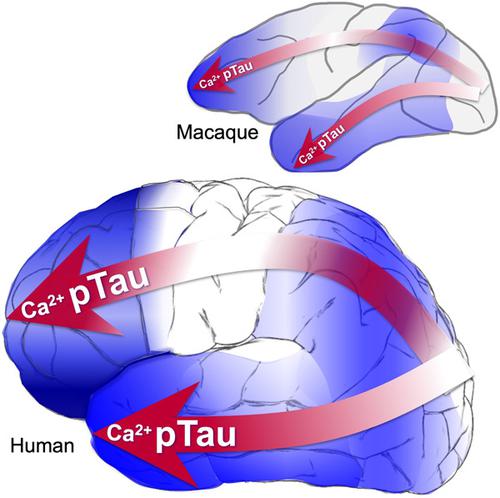
Tau pathology in Alzheimer's disease (AD) preferentially afflicts the limbic and recently enlarged association cortices, causing a progression of mnemonic and cognitive deficits. Although genetic mouse models have helped reveal mechanisms underlying the rare, autosomal-dominant forms of AD, the etiology of the more common, sporadic form of AD remains unknown, and is challenging to study in mice due to their limited association cortex and lifespan. It is also difficult to study in human brains, as early-stage tau phosphorylation can degrade postmortem. In contrast, rhesus monkeys have extensive association cortices, are long-lived, and can undergo perfusion fixation to capture early-stage tau phosphorylation in situ. Most importantly, rhesus monkeys naturally develop amyloid plaques, neurofibrillary tangles comprised of hyperphosphorylated tau, synaptic loss, and cognitive deficits with advancing age, and thus can be used to identify the early molecular events that initiate and propel neuropathology in the aging association cortices. Studies to date suggest that the particular molecular signaling events needed for higher cognition—for example, high levels of calcium to maintain persistent neuronal firing- lead to tau phosphorylation and inflammation when dysregulated with advancing age. The expression of NMDAR-NR2B (GluN2B)—the subunit that fluxes high levels of calcium—increases over the cortical hierarchy and with the expansion of association cortex in primate evolution, consistent with patterns of tau pathology. In the rhesus monkey dorsolateral prefrontal cortex, spines contain NMDAR-NR2B and the molecular machinery to magnify internal calcium release near the synapse, as well as phosphodiesterases, mGluR3, and calbindin to regulate calcium signaling. Loss of regulation with inflammation and/or aging appears to be a key factor in initiating tau pathology. The vast expansion in the numbers of these synapses over primate evolution is consistent with the degree of tau pathology seen across species: marmoset < rhesus monkey < chimpanzee < human, culminating in the vast neurodegeneration seen in humans with AD.
中文翻译:

衰老非人类灵长类动物的研究阐明了早期阿尔茨海默病样神经病理学的病因:进化的观点
阿尔茨海默病 (AD) 中的 Tau 病理学优先影响边缘和最近扩大的关联皮质,导致记忆和认知缺陷的进展。尽管遗传小鼠模型有助于揭示罕见的常染色体显性 AD 形式的潜在机制,但更常见的散发形式 AD 的病因仍然未知,并且由于其关联皮层和寿命有限,因此在小鼠中进行研究具有挑战性。在人脑中进行研究也很困难,因为早期的 tau 磷酸化会在死后降解。相比之下,恒河猴具有广泛的关联皮质,寿命长,并且可以进行灌注固定以在原位捕获早期阶段的 tau 磷酸化。最重要的是,恒河猴自然会形成淀粉样斑块、由过度磷酸化的 tau 蛋白组成的神经原纤维缠结,突触丢失和随着年龄增长的认知缺陷,因此可用于识别在衰老关联皮质中启动和推动神经病理学的早期分子事件。迄今为止的研究表明,高级认知所需的特定分子信号事件(例如,维持持续神经元放电的高水平钙)会导致 tau 磷酸化和炎症随着年龄的增长而失调。NMDAR-NR2B (GluN2B)——一种促进高水平钙流动的亚基——的表达随着灵长类动物进化中皮质层次结构和关联皮质的扩张而增加,这与 tau 病理学模式一致。在恒河猴背外侧前额叶皮层中,刺含有 NMDAR-NR2B 和放大突触附近内部钙释放的分子机制,以及磷酸二酯酶、mGluR3 和钙结合蛋白,以调节钙信号。炎症和/或衰老导致的调节丧失似乎是引发 tau 病理的关键因素。这些突触数量在灵长类动物进化过程中的巨大扩张与跨物种观察到的 tau 病理程度一致:狨 < 恒河猴 < 黑猩猩 < 人类,最终导致在患有 AD 的人类中出现大量神经变性。
更新日期:2021-05-07
中文翻译:

衰老非人类灵长类动物的研究阐明了早期阿尔茨海默病样神经病理学的病因:进化的观点
阿尔茨海默病 (AD) 中的 Tau 病理学优先影响边缘和最近扩大的关联皮质,导致记忆和认知缺陷的进展。尽管遗传小鼠模型有助于揭示罕见的常染色体显性 AD 形式的潜在机制,但更常见的散发形式 AD 的病因仍然未知,并且由于其关联皮层和寿命有限,因此在小鼠中进行研究具有挑战性。在人脑中进行研究也很困难,因为早期的 tau 磷酸化会在死后降解。相比之下,恒河猴具有广泛的关联皮质,寿命长,并且可以进行灌注固定以在原位捕获早期阶段的 tau 磷酸化。最重要的是,恒河猴自然会形成淀粉样斑块、由过度磷酸化的 tau 蛋白组成的神经原纤维缠结,突触丢失和随着年龄增长的认知缺陷,因此可用于识别在衰老关联皮质中启动和推动神经病理学的早期分子事件。迄今为止的研究表明,高级认知所需的特定分子信号事件(例如,维持持续神经元放电的高水平钙)会导致 tau 磷酸化和炎症随着年龄的增长而失调。NMDAR-NR2B (GluN2B)——一种促进高水平钙流动的亚基——的表达随着灵长类动物进化中皮质层次结构和关联皮质的扩张而增加,这与 tau 病理学模式一致。在恒河猴背外侧前额叶皮层中,刺含有 NMDAR-NR2B 和放大突触附近内部钙释放的分子机制,以及磷酸二酯酶、mGluR3 和钙结合蛋白,以调节钙信号。炎症和/或衰老导致的调节丧失似乎是引发 tau 病理的关键因素。这些突触数量在灵长类动物进化过程中的巨大扩张与跨物种观察到的 tau 病理程度一致:狨 < 恒河猴 < 黑猩猩 < 人类,最终导致在患有 AD 的人类中出现大量神经变性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号