Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-05-07 , DOI: 10.1016/j.jhazmat.2021.126043 Wei Zou 1 , Zhenzhen Liu 1 , Rui Li 1 , Caixia Jin 1 , Xingli Zhang 1 , Kai Jiang 1
|
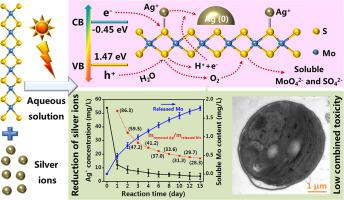
The transformation of Ag+ is strongly correlated with its risks in aquatic environment. Considering the wide application of molybdenum disulfide (MoS2) and the inevitable release into the environment, the effects of MoS2 on Ag+ transformation and toxicity are of great concerns. This study revealed the pH−dependent reduction of Ag+ (0.5 mM) to Ag nanoparticles (AgNPs) by MoS2 (50 mg/L) and solar irradiation obviously accelerates the AgNPs formation (2.638 mg/L per day, pH=7.0) compared with dark condition (0.637 mg/L per day), ascribing to the electrons capture from electron−hole pairs of MoS2 by Ag+. Ionic strengths and natural organic matter decreased the AgNPs yield. Metallic 1 T phase of MoS2 primarily participated in AgNPs formation and was oxidized to soluble ions (MoO42−) due to the oxygen generation in valance band. The above processes also occurred between Ag+ and MoS2 at environmentally relevant concentrations. Further, photoinduced transformation of Ag+ by MoS2 (10−100 μg/L) significantly lowered its toxicity to freshwater algae. The AgNPs formation on MoS2 reduced the bioavailability of Ag+ to algae, which was the mechanism for attenuated Ag+ toxicity. The provided data are helpful for better understanding the roles of MoS2 on the environmental fates and risks of metal ions under natural conditions.
中文翻译:

在环境相关浓度下,二硫化钼纳米片对银离子的光诱导转化减弱了其对淡水藻类的毒性
Ag +的转化与其在水生环境中的风险密切相关。考虑到二硫化钼(MoS 2)的广泛应用和不可避免的释放到环境中,MoS 2对Ag +转化和毒性的影响备受关注。该研究表明,MoS 2 (50 mg/L) 和太阳照射明显加速了 AgNPs 的形成(每天 2.638 mg/L,pH=7.0),Ag + (0.5 mM) 到 Ag 纳米颗粒 (AgNPs)的 pH 依赖性还原与黑暗条件(每天0.637 mg/L)相比,归因于Ag从 MoS 2的电子-空穴对中捕获电子 +。离子强度和天然有机物降低了 AgNPs 的产量。 MoS 2 的金属 1 T 相主要参与 AgNPs 的形成,并由于价带中的氧生成而被氧化为可溶性离子 (MoO 4 2- )。上述过程也在环境相关浓度的Ag +和 MoS 2之间发生。此外,MoS 2 (10-100 μg/L)对Ag +的光诱导转化显着降低了其对淡水藻类的毒性。在 MoS 2上形成的 AgNPs降低了 Ag +对藻类的生物利用度,这是衰减 Ag +的机制 毒性。所提供的数据有助于更好地理解 MoS 2在自然条件下对环境归宿和金属离子风险的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号