Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-05-07 , DOI: 10.1016/j.jhazmat.2021.126009 Lin Liu 1 , Siong Fong Sim 2 , Sen Lin 1 , Jiang Wan 1 , Wei Zhang 1 , Qiannan Li 1 , Cheng Peng 1
|
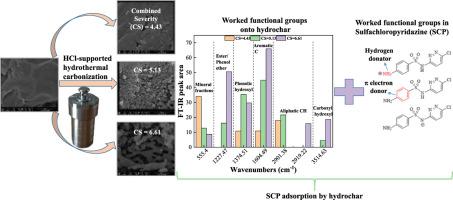
In this study, various HCl-supported hydrochar made from root powder of long-root Eichhornia crassipes were applied to adsorb aqueous sulfachloropyridazine (SCP). Adsorption capacity (qe μg g-1) was positively correlated with combined severity-CS. With CS increasing, carbonization degree, hydrophobicity, porosity and isoelectric point of hydrochar increased, but content of polar functional groups decreased. Hydrophobic interaction was important for SCP adsorption. A 24×36 peak area table was generated from 24 FT-IR absorbance spectra computed by peak detection algorithm. Afterwards, correlation analysis between qe μg g-1 and FT-IR peak area were conducted, indicating that wavenumbers at 555.4, 1227.47, 1374.51, 1604.5, 2901.4/2919.2 and 3514.63 cm-1 were helpful for SCP adsorption. Further, multivariate linear regression analyses showed that aromatic skeleton and phenolic hydroxyl were the two biggest contributors. Electrostatic attraction did not exist during the SCP adsorption process. Under strong acid condition, protonated amino groups in cationic SCP acting as a hydrogen donator interacted with electron-rich functional groups onto hydrochar by Hydrogen interaction. Under weak acid condition, neutral SCP served as an π electron donor to bond with hydrochar by π-π electron donator-acceptor interaction. This work could guide the functional groups modification strategy of hydrochar to make better use of it in water purification field.
中文翻译:

HCl负载烃的综合结构和化学分析及其对磺胺氯哒嗪水溶液的吸附机理
在这项研究中,由长根凤眼莲根粉制成的各种HCl负载的碳氢化合物被用于吸附磺胺氯哒嗪水溶液(SCP)。吸附容量(Q Ë微克克-1)呈正组合的严重性CS相关联。随着CS的增加,炭化程度,疏水性,孔隙率和等电点增加,但极性官能团的含量下降。疏水相互作用对SCP的吸收很重要。由通过峰检测算法计算的24个FT-IR吸收光谱生成了24×36峰面积表。然后,Q之间的相关性分析Ë微克克-1进行了FT-IR峰面积分析,表明波数分别为555.4、1227.47、1374.51、1604.5、2901.4 / 2919.2和3514.63 cm -1对SCP的吸收有帮助。此外,多元线性回归分析表明,芳香族骨架和酚羟基是两个最大的贡献者。SCP吸附过程中不存在静电吸引。在强酸条件下,阳离子SCP中作为氢供体的质子化氨基与富电子官能团通过氢相互作用与烃类相互作用。在弱酸条件下,中性SCP充当π电子供体,通过π-π电子供体-受体相互作用与水碳键结合。这项工作可以指导水炭的官能团修饰策略,使其在净水领域得到更好的利用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号