Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-05-07 , DOI: 10.1016/j.jhazmat.2021.125995 So Yeon Yoon 1 , Seok Byum Jang 1 , Kien Tiek Wong 1 , Hyeseong Kim 1 , Min Ji Kim 1 , Choe Earn Choong 1 , Jae-Kyu Yang 1 , Yoon-Young Chang 1 , Sang-Eun Oh 2 , Yeomin Yoon 3 , Min Jang 1
|
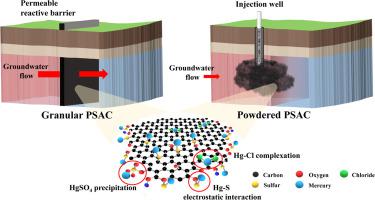
This study utilized a facile and scalable one-pot wet impregnation method for Hg(II) adsorption to prepare sulfur-anchored palm shell waste activated carbon powder (PSAC-S). The experimental results revealed that the sulfur precursors promote the surface charge on the PSAC and enhance Hg(II) removal via the Na2S > Na2S2O4 > CH3CSNH2 sequence. PSAC-S prepared using Na2S had significant Hg(II) sorption efficiencies, achieving a maximum sorption capacity of 136 mg g−1 from the Freundlich model. Compared to PSAC, PSAC-S had an enhancement in Hg(II) sorption behavior for heterogeneous interactions with sulfur. PSAC-S also demonstrated high Hg(II) sorption capacities over a wide range of solution pH, while ionic strength had an insignificant impact on Hg(II) removal efficiencies. Through various spectroscopic analyses, we identified the mechanisms of Hg(II) removal by PSAC-S as electrostatic interactions, Hg-Cl complexation, and precipitation as HgSO4. Moreover, PSAC-S unveiled high adsorption affinity and Hg(II) stability in actual groundwater (even in µg L−1 level). These overall results show the potentials of PSAC-S as an alternative, easily scalable material for in-situ Hg(II) remediation.
中文翻译:

硫锚定的棕榈壳废料基活性炭,可超高吸附Hg(II),用于原位地下水处理
这项研究利用一种简便且可扩展的一锅湿法浸渍Hg(II)的方法来制备硫磺锚定的棕榈壳废活性炭粉(PSAC-S)。实验结果表明,硫前体通过Na 2 S> Na 2 S 2 O 4 > CH 3 CSNH 2序列促进PSAC上的表面电荷并增强Hg(II)的去除。使用Na 2 S制备的PSAC-S具有显着的Hg(II)吸附效率,最大吸附容量为136 mg g -1来自Freundlich模型。与PSAC相比,PSAC-S的Hg(II)吸附行为增强,可与硫发生异质相互作用。PSAC-S还显示了在溶液pH的宽范围内都具有很高的Hg(II)吸附能力,而离子强度对Hg(II)的去除效率影响不大。通过各种光谱分析,我们确定了PSAC-S去除Hg(II)的机理为静电相互作用,Hg-Cl络合和沉淀为HgSO 4。此外,PSAC-S在实际地下水中(甚至在µg L -1水平下)还具有很高的吸附亲和力和Hg(II)稳定性。这些总体结果表明,PSAC-S作为替代性,易于扩展的原位Hg(II)修复材料的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号