Current Organic Synthesis ( IF 1.7 ) Pub Date : 2021-07-31 , DOI: 10.2174/1570179418666210113160949 Arturo R M Salgado 1 , Carlos E P Galvis 2 , Vladimir V Kouznetsov 2 , Carlos Mario Meléndez 1
|
Background: Hexahydro-2H-pyrano[3,2-c]quinolines are known to have antibacterial, antifungal, and antitumor properties. Great efforts have been made to develop new synthetic methods that lead to the synthesis of valuable libraries. Extensive methodologies, low yields, excessive amounts of catalyst and expensive reactants are some of the limitations of current methodologies.
Aims and Objective: Developing a useful and efficient method to construct diversely substituted hexahydro-2Hpyrano[ 3,2-c]quinolines into good to excellent yields through a cationic imino-Diels-Alder/N-debenzylation methodology.
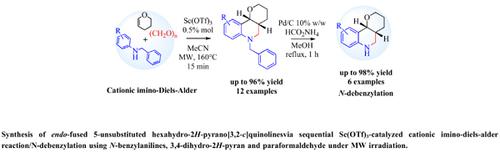
Method: The cationic imino-Diels-Alder/N-debenzylation methodology was used for the preparation of substituted hexahydro-2H-pyrano[3,2-c]quinolines. It involves the use of Sc(OTf)3 for activation of cationic imino- Diels-Alder cycloaddition reaction of N-benzylanilines, 3,4-dihydro-2H-pyran and paraformaldehyde in MeCN; and microwave irradiation to shorten reaction time to afford new 6-benzyl-hexahydro-2H-pyrano[3,2- c]quinolines whose catalytic transfer debenzylation reactions with HCO2NH4 in the presence of Pd/C (10%) and methanol give the new 5-unsubstituted pyrano[3,2-c]quinolines in excellent yields.
Results: We found that optimal conditions for the preparation of hexahydro-2H-pyrano[3,2-c]quinolines were Sc(OTf)3 0.5 % and acetonitrile at 160°C for 15 min; and using paraformaldehyde obtained the 6-benzylhexahydro- 2H-pyrano [3,2-c]quinolines with excellent yields, while the N-debenzylation process using ammonium formate in the presence of Pd/C and methanol resulted in the synthesis of hexahydro-2H-pyrano [3,2-c] quinolines with quantitative yields (95-98%).
Conclusion: We describe an efficient method to synthesize hexahydro-2H-pyrano[3,2-c]quinolines via the cationic imino-Diels-Alder/N-debenzylation methodology using Sc(OTf)3 0.5 % as Lewis Acid catalyst. Excellent yields of the products, use of MW irradiation, short times of reactions, and an efficient and highly diversified method are some of the main advantages of this new protocol.
中文翻译:

通过顺序 Sc(OTf)3-催化阳离子亚氨基-Diels-Alder 反应/使用 N-苄基苯胺、3,4-二氢的 N-脱苄基化合成内稠合 5-未取代的六氢-2H-吡喃并[3,2-c]喹啉-2H-吡喃和多聚甲醛在MW辐照下
背景:已知六氢-2H-吡喃并[3,2-c]喹啉具有抗菌、抗真菌和抗肿瘤特性。已经付出了巨大努力来开发新的合成方法,从而合成有价值的文库。广泛的方法、低产率、过量的催化剂和昂贵的反应物是当前方法的一些限制。
目的和目的:开发一种有用且有效的方法,通过阳离子亚氨基-Diels-Alder/N-脱苄方法将多种取代的六氢-2H吡喃并[3,2-c]喹啉构建成良好至极好的产率。
方法:采用阳离子亚氨基-Diels-Alder/N-脱苄方法制备取代的六氢-2H-吡喃并[3,2-c]喹啉。它涉及使用 Sc(OTf) 3来活化 MeCN 中 N-苄基苯胺、3,4-二氢-2H-吡喃和多聚甲醛的阳离子亚氨基-Diels-Alder 环加成反应;和微波辐射以缩短反应时间以提供新的 6-苄基-六氢-2H-吡喃并[3,2-c]喹啉,其在 Pd/C (10%) 和甲醇存在下与 HCO 2 NH 4发生催化转移脱苄反应以优异的收率得到新的 5-未取代的吡喃并[3,2-c]喹啉。
结果:我们发现制备六氢-2H-吡喃并[3,2-c]喹啉的最佳条件是Sc(OTf) 3 0.5 % 和乙腈在160°C 下15 min;并使用多聚甲醛以优异的收率获得了 6-苄基六氢-2H-吡喃并 [3,2-c] 喹啉,而在 Pd/C 和甲醇存在下使用甲酸铵的 N-脱苄基过程导致合成六氢-2H -吡喃并 [3,2-c] 喹啉的定量收率 (95-98%)。
结论:我们描述了一种使用 Sc(OTf)3 0.5% 作为路易斯酸催化剂,通过阳离子亚氨基-Diels-Alder/N-脱苄基化方法合成六氢-2H-吡喃并[3,2-c]喹啉的有效方法。产品的出色产量、使用 MW 辐射、反应时间短以及高效且高度多样化的方法是该新协议的一些主要优点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号