Current Organic Synthesis ( IF 1.7 ) Pub Date : 2021-05-31 , DOI: 10.2174/1570179417666201231110306 Shashika Sevvandi Perera 1 , Umayangani Kumari Wanninayake 1 , Dhanushi Thathsara Welideniya 1 , Adeesha Saseenda Jayathilaka 1 , Anjana Delpe Acharige 1 , Upamalika Samanthi 1 , Shihan Shalinda Kaleel 1 , Veranja Karunaratne 1 , Gehan Amaratunga 1 , Dinara Shashanka Gunasekera 1
|
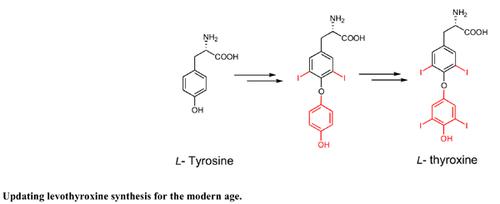
Synthesis of levothyroxine sodium, the sodium salt of a synthetic levoisomer of thyroxine, revolutionized the management of hypothyroidism and related symptoms. However, the primary synthetic route to this active pharmaceutical ingredient (API) is more than 70+ years old with low-yielding steps and obsolete reagents. It lacks experimental data on intermediates, making laboratory and large-scale synthesis of this API difficult and time-consuming. Here, we describe an improved synthesis of levothyroxine using commonly available modern reagents. By modifying and replacing low yielding and/or unproductive steps of Chalmers synthesis, we were able to achieve higher overall yields (39-51%) consistently. Key modifications include an alternative path to the selective N-acetylation step that yielded 5 in a pure and consistent fashion. Our improved methodology, coupled with detailed experimental data, provides a practical alternative to existing methods that can be conveniently implemented to synthesize Levothyroxine sodium in fine chemical settings.
中文翻译:

更新现代左旋甲状腺素合成
左旋甲状腺素钠的合成,甲状腺素的合成左旋异构体的钠盐,彻底改变了甲状腺功能减退症和相关症状的管理。然而,这种活性药物成分 (API) 的主要合成路线已有 70 多年的历史,其步骤产量低且试剂过时。它缺乏中间体的实验数据,使得这种 API 的实验室和大规模合成变得困难和耗时。在这里,我们描述了使用常用的现代试剂改进左旋甲状腺素的合成。通过修改和替换 Chalmers 合成的低产率和/或非生产性步骤,我们能够始终如一地实现更高的总产率 (39-51%)。关键修改包括选择性 N-乙酰化步骤的替代路径,该步骤以纯净和一致的方式产生 5。











































 京公网安备 11010802027423号
京公网安备 11010802027423号