当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and functional studies of SAV1707 from Staphylococcus aureus elucidate its distinct metal‐dependent activity and a crucial residue for catalysis
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-05-05 , DOI: 10.1107/s2059798321001923 Dong Gyun Kim 1 , Kyu Yeon Lee 1 , Sang Jae Lee 2 , Seung Ho Cheon 1 , Yuri Choi 3 , Hyung Ho Lee 3 , Hee Chul Ahn 4 , Bong Jin Lee 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-05-05 , DOI: 10.1107/s2059798321001923 Dong Gyun Kim 1 , Kyu Yeon Lee 1 , Sang Jae Lee 2 , Seung Ho Cheon 1 , Yuri Choi 3 , Hyung Ho Lee 3 , Hee Chul Ahn 4 , Bong Jin Lee 1
Affiliation
|
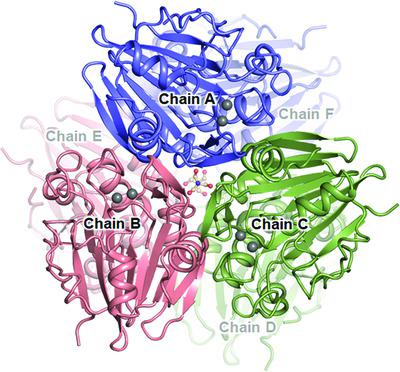
The metallo‐β‐lactamase fold is the most abundant metal‐binding domain found in two major kingdoms: bacteria and archaea. Despite the rapid growth in genomic information, most of these enzymes, which may play critical roles in cellular metabolism, remain uncharacterized in terms of structure and function. In this study, X‐ray crystal structures of SAV1707, a hypothetical metalloenzyme from Staphylococcus aureus, and its complex with cAMP are reported at high resolutions of 2.05 and 1.55 Å, respectively, with a detailed atomic description. Through a functional study, it was verified that SAV1707 has Ni2+‐dependent phosphodiesterase activity and Mn2+‐dependent endonuclease activity, revealing a different metal selectivity depending on the reaction. In addition, the crystal structure of cAMP‐bound SAV1707 shows a unique snapshot of cAMP that reveals the binding mode of the intermediate, and a key residue Phe511 that forms π–π interactions with cAMP was verified as contributing to substrate recognition by functional studies of its mutant. Overall, these findings characterized the relationship between the structure and function of SAV1707 and may provide further understanding of metalloenzymes possessing the metallo‐β‐lactamase fold.
中文翻译:

来自金黄色葡萄球菌的 SAV1707 的结构和功能研究阐明了其独特的金属依赖性活性和催化的关键残基
金属-β-内酰胺酶折叠是在两个主要王国中发现的最丰富的金属结合域:细菌和古细菌。尽管基因组信息快速增长,但这些可能在细胞代谢中起关键作用的酶中的大多数在结构和功能方面仍然没有特征。在这项研究中,SAV1707(一种来自金黄色葡萄球菌的假设金属酶)及其与 cAMP 的复合物的X 射线晶体结构分别以 2.05 和 1.55 Å 的高分辨率报告,并提供了详细的原子描述。通过功能研究,证实 SAV1707 具有 Ni 2+依赖性磷酸二酯酶活性和 Mn 2+依赖的核酸内切酶活性,根据反应显示不同的金属选择性。此外,与 cAMP 结合的 SAV1707 的晶体结构显示了 cAMP 的独特快照,揭示了中间体的结合模式,并且通过功能研究证实了与 cAMP 形成 π-π 相互作用的关键残基 Phe511 有助于底物识别它的突变体。总的来说,这些发现表征了 SAV1707 的结构和功能之间的关系,并可能提供对具有金属-β-内酰胺酶折叠的金属酶的进一步理解。
更新日期:2021-05-06
中文翻译:

来自金黄色葡萄球菌的 SAV1707 的结构和功能研究阐明了其独特的金属依赖性活性和催化的关键残基
金属-β-内酰胺酶折叠是在两个主要王国中发现的最丰富的金属结合域:细菌和古细菌。尽管基因组信息快速增长,但这些可能在细胞代谢中起关键作用的酶中的大多数在结构和功能方面仍然没有特征。在这项研究中,SAV1707(一种来自金黄色葡萄球菌的假设金属酶)及其与 cAMP 的复合物的X 射线晶体结构分别以 2.05 和 1.55 Å 的高分辨率报告,并提供了详细的原子描述。通过功能研究,证实 SAV1707 具有 Ni 2+依赖性磷酸二酯酶活性和 Mn 2+依赖的核酸内切酶活性,根据反应显示不同的金属选择性。此外,与 cAMP 结合的 SAV1707 的晶体结构显示了 cAMP 的独特快照,揭示了中间体的结合模式,并且通过功能研究证实了与 cAMP 形成 π-π 相互作用的关键残基 Phe511 有助于底物识别它的突变体。总的来说,这些发现表征了 SAV1707 的结构和功能之间的关系,并可能提供对具有金属-β-内酰胺酶折叠的金属酶的进一步理解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号