当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Sulfide Solubility in Aqueous N-Methylpyrrolidone Solution
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-05-05 , DOI: 10.1021/acs.jced.0c00923 Mohammad Shokouhi 1 , Amir Hossein Jalili 1 , Ensieh Ganji Babakhani 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-05-05 , DOI: 10.1021/acs.jced.0c00923 Mohammad Shokouhi 1 , Amir Hossein Jalili 1 , Ensieh Ganji Babakhani 1
Affiliation
|
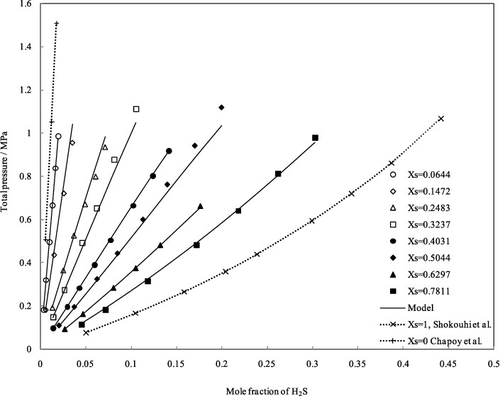
Hydrogen sulfide (H2S) solubility was experimentally quantified in aqueous N-methylpyrrolidone (NMP) solution. Mixtures of (NMP + H2O) were established in the whole composition range of x̃NMP = (0.0644–1.000), operational temperatures range of 303.15–353.15 K, and vapor pressure of mixed solvents up to about 1.3 MPa. The reported experimental data show that as a common result of gas absorption in liquids, the H2S solubility in mixed solvents increases with increasing pressure and decreasing temperature and the mole fraction of NMP in gas-free (NMP + H2O) solutions has a positive influence on H2S solubility. Modeling of experimental data was done using Gibbs excess energy of mixed solvent. The average and maximum relative percent deviation of correlated values are 2.56 and 9.71%, respectively, from 301 obtained experimental data.
中文翻译:

硫化氢在N-甲基吡咯烷酮水溶液中的溶解度
在N-甲基吡咯烷酮(NMP)水溶液中通过实验确定了硫化氢(H 2 S)的溶解度。(NMP + H的混合物2 O)建立在整个组合物范围X NMP =(0.0644-1.000),操作温度范围303.15-353.15钾,和混合溶剂的蒸气压高达约1.3兆帕。报告的实验数据表明,作为气体在液体中吸收的常见结果,混合溶剂中的H 2 S溶解度随压力和温度降低而增加,并且无气体(NMP + H 2 O)溶液中NMP的摩尔分数为对H 2有积极影响S的溶解度。实验数据的建模是使用Gibbs混合溶剂的多余能量完成的。从301个获得的实验数据中,相关值的平均和最大相对百分比偏差分别为2.56%和9.71%。
更新日期:2021-05-13
中文翻译:

硫化氢在N-甲基吡咯烷酮水溶液中的溶解度
在N-甲基吡咯烷酮(NMP)水溶液中通过实验确定了硫化氢(H 2 S)的溶解度。(NMP + H的混合物2 O)建立在整个组合物范围X NMP =(0.0644-1.000),操作温度范围303.15-353.15钾,和混合溶剂的蒸气压高达约1.3兆帕。报告的实验数据表明,作为气体在液体中吸收的常见结果,混合溶剂中的H 2 S溶解度随压力和温度降低而增加,并且无气体(NMP + H 2 O)溶液中NMP的摩尔分数为对H 2有积极影响S的溶解度。实验数据的建模是使用Gibbs混合溶剂的多余能量完成的。从301个获得的实验数据中,相关值的平均和最大相对百分比偏差分别为2.56%和9.71%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号