当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-4-16 , DOI: 10.1039/d1ee00545f Panpan Li 1, 2, 3, 4, 5 , Zhaoyu Jin 3, 4, 5, 6, 7 , Zhiwei Fang 1, 2, 3, 4, 5 , Guihua Yu 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-4-16 , DOI: 10.1039/d1ee00545f Panpan Li 1, 2, 3, 4, 5 , Zhaoyu Jin 3, 4, 5, 6, 7 , Zhiwei Fang 1, 2, 3, 4, 5 , Guihua Yu 1, 2, 3, 4, 5
Affiliation
|
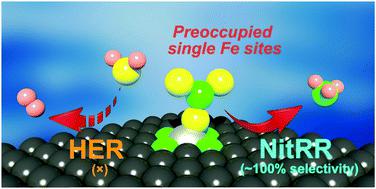
The necessity to pursue sustainable ammonia (NH3) production with economic and environment-friendly technologies is growing with the global development aim of future fertilizer and renewable energy industries. Electrosynthesis of ammonia from nitrate reduction is encouraging for both environmental nitrogen pollution management and artificial nutrient recycling. However, it is fundamentally difficult to regulate the reaction pathways for efficient and selective ammonia production over competing reactions, e.g., the hydrogen evolution reaction, particularly under aqueous conditions. Enlightened by the unique and tunable local electronic structures, an iron-based single-atom catalyst is reported in this contribution. We demonstrate a polymer-hydrogel strategy for preparing nitrogen-coordinated Fe sites with uniform atomic dispersion on carbon. The catalyst exhibits a maximum NH3 yield rate of 2.75 mgNH3 h−1 cm−2 (ca. 30 molNH3 h−1 gFe−1) with nearly 100% faradaic efficiency. Furthermore, the catalytically active individual Fe site in the isolated atom state displays a twelve times higher turnover frequency than that in metallic Fe nanoparticles. Experimental evidence suggests that the single-site iron would experience a nitrate-preoccupied transition center, which prohibits water adsorption as the competitive reaction that generally exists for bulk catalysts. Theoretical insights into the localized structure further assist in better understanding and support of the high selectivity for NH3 achieved by the Fe single-atom catalyst.
中文翻译:

具有活动中心的单中心铁催化剂,可实现硝酸盐选择性合成氨
随着未来肥料和可再生能源行业的全球发展目标,采用经济和环境友好型技术进行可持续氨(NH 3)生产的必要性正在增长。从硝酸盐还原中电合成氨对于环境氮污染管理和人工营养物回收都是令人鼓舞的。然而,从根本上来说,很难调节反应途径,以在竞争性反应(例如竞争性反应)中高效,选择性地生产氨,特别是在水性条件下发生氢释放反应。据报道,这种独特的可调谐的局部电子结构启发了铁基单原子催化剂。我们展示了一种聚合物-水凝胶策略,该策略可制备在碳上具有均匀原子分散性的氮配位铁位点。该催化剂的最大NH 3产率为2.75 mg NH 3 h -1 cm -2(约30 mol NH 3 h -1 g Fe -1)的法拉第效率将近100%。此外,处于孤立原子状态的具有催化活性的单个Fe位点的翻转频率比金属Fe纳米颗粒的翻转频率高12倍。实验证据表明,单中心铁将经历一个硝酸盐占据的过渡中心,该过渡中心禁止水吸附,这是本体催化剂通常存在的竞争性反应。对局部结构的理论见解进一步有助于更好地理解和支持由Fe单原子催化剂实现的对NH 3的高选择性。
更新日期:2021-05-06
中文翻译:

具有活动中心的单中心铁催化剂,可实现硝酸盐选择性合成氨
随着未来肥料和可再生能源行业的全球发展目标,采用经济和环境友好型技术进行可持续氨(NH 3)生产的必要性正在增长。从硝酸盐还原中电合成氨对于环境氮污染管理和人工营养物回收都是令人鼓舞的。然而,从根本上来说,很难调节反应途径,以在竞争性反应(例如竞争性反应)中高效,选择性地生产氨,特别是在水性条件下发生氢释放反应。据报道,这种独特的可调谐的局部电子结构启发了铁基单原子催化剂。我们展示了一种聚合物-水凝胶策略,该策略可制备在碳上具有均匀原子分散性的氮配位铁位点。该催化剂的最大NH 3产率为2.75 mg NH 3 h -1 cm -2(约30 mol NH 3 h -1 g Fe -1)的法拉第效率将近100%。此外,处于孤立原子状态的具有催化活性的单个Fe位点的翻转频率比金属Fe纳米颗粒的翻转频率高12倍。实验证据表明,单中心铁将经历一个硝酸盐占据的过渡中心,该过渡中心禁止水吸附,这是本体催化剂通常存在的竞争性反应。对局部结构的理论见解进一步有助于更好地理解和支持由Fe单原子催化剂实现的对NH 3的高选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号