当前位置:
X-MOL 学术
›
Arch. Insect Biochem. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intestinal proteases profiling from Anticarsia gemmatalis and their binding to inhibitors
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2021-05-05 , DOI: 10.1002/arch.21792 Neilier R Silva-Júnior 1 , Yaremis M Cabrera 1 , Samuel L Barbosa 1 , Rafael de A Barros 1 , Edvaldo Barros 2 , Camilo E Vital 1 , Humberto J O Ramos 1, 2 , Maria Goreti A Oliveira 1
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2021-05-05 , DOI: 10.1002/arch.21792 Neilier R Silva-Júnior 1 , Yaremis M Cabrera 1 , Samuel L Barbosa 1 , Rafael de A Barros 1 , Edvaldo Barros 2 , Camilo E Vital 1 , Humberto J O Ramos 1, 2 , Maria Goreti A Oliveira 1
Affiliation
|
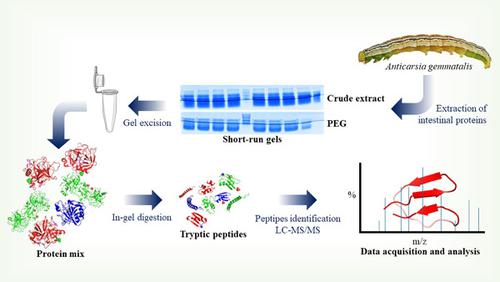
Although the importance of intestinal hydrolases is recognized, there is little information on the intestinal proteome of lepidopterans such as Anticarsia gemmatalis. Thus, we carried out the proteomic analysis of the A. gemmatalis intestine to characterize the proteases by LC/MS. We examined the interactions of proteins identified with protease inhibitors (PI) using molecular docking. We found 54 expressed antigens for intestinal protease, suggesting multiple important isoforms. The hydrolytic arsenal featured allows for a more comprehensive understanding of insect feeding. The docking analysis showed that the soybean PI (SKTI) could bind efficiently with the trypsin sequences and, therefore, insect resistance does not seem to involve changing the sequences of the PI binding site. In addition, a SERPIN was identified and the interaction analysis showed the inhibitor binding site is in contact with the catalytic site of trypsin, possibly acting as a regulator. In addition, this SERPIN and the identified PI sequences can be targets for the control of proteolytic activity in the caterpillar intestine and serve as a support for the rational design of a molecule with greater stability, less prone to cleavage by proteases and viable for the control of insect pests such as A. gemmatalis.
中文翻译:

Anticarsia gemmatalis 的肠道蛋白酶谱及其与抑制剂的结合
尽管肠道水解酶的重要性已得到认可,但关于鳞翅目昆虫(如Anticarsia gemmatalis)的肠道蛋白质组的信息却很少。因此,我们对A. gemmatalis进行了蛋白质组学分析通过 LC/MS 表征蛋白酶。我们使用分子对接检查了用蛋白酶抑制剂 (PI) 鉴定的蛋白质的相互作用。我们发现了 54 种表达的肠道蛋白酶抗原,表明存在多种重要的同种型。水解武器库的特点允许更全面地了解昆虫喂养。对接分析表明,大豆 PI (SKTI) 可以与胰蛋白酶序列有效结合,因此,昆虫抗性似乎不涉及改变 PI 结合位点的序列。此外,鉴定了 SERPIN,相互作用分析表明抑制剂结合位点与胰蛋白酶的催化位点接触,可能充当调节剂。此外,A. gemmatalis。
更新日期:2021-06-23
中文翻译:

Anticarsia gemmatalis 的肠道蛋白酶谱及其与抑制剂的结合
尽管肠道水解酶的重要性已得到认可,但关于鳞翅目昆虫(如Anticarsia gemmatalis)的肠道蛋白质组的信息却很少。因此,我们对A. gemmatalis进行了蛋白质组学分析通过 LC/MS 表征蛋白酶。我们使用分子对接检查了用蛋白酶抑制剂 (PI) 鉴定的蛋白质的相互作用。我们发现了 54 种表达的肠道蛋白酶抗原,表明存在多种重要的同种型。水解武器库的特点允许更全面地了解昆虫喂养。对接分析表明,大豆 PI (SKTI) 可以与胰蛋白酶序列有效结合,因此,昆虫抗性似乎不涉及改变 PI 结合位点的序列。此外,鉴定了 SERPIN,相互作用分析表明抑制剂结合位点与胰蛋白酶的催化位点接触,可能充当调节剂。此外,A. gemmatalis。










































 京公网安备 11010802027423号
京公网安备 11010802027423号