Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Polyelectrolytes with Proteins: Quantifying the Role of Water
Advanced Science ( IF 14.3 ) Pub Date : 2021-05-03 , DOI: 10.1002/advs.202100661 Jacek J Walkowiak 1, 2 , Matthias Ballauff 1
Advanced Science ( IF 14.3 ) Pub Date : 2021-05-03 , DOI: 10.1002/advs.202100661 Jacek J Walkowiak 1, 2 , Matthias Ballauff 1
Affiliation
|
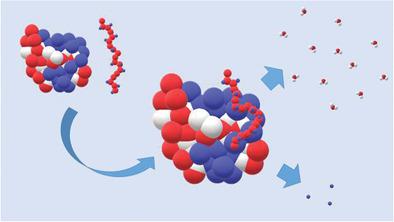
A theoretical model is presented for the free energy ΔGb of complex formation between a highly charged polyelectrolyte and a protein. The model introduced here comprises both the effect of released counterions and the uptake or release of water molecules during complex formation. The resulting expression for ΔGb is hence capable of describing the dependence of ΔGb on temperature as well as on the concentration of salt in the system: An increase of the salt concentration in the solution increases the activity of the ions and counterion release becomes less effective for binding. On the other hand, an increased salt concentration leads to the decrease of the activity of water in bulk. Hence, release of water molecules during complex formation will be more advantageous and lead to an increase of the magnitude of ΔGb and the binding constant. It is furthermore demonstrated that the release or uptake of water molecules is the origin of the marked enthalpy–entropy cancellation observed during complex formation of polyelectrolytes with proteins. The comparison with experimental data on complex formation between a synthetic (sulfated dendritic polyglycerol) and natural polyelectrolytes (DNA; heparin) with proteins shows full agreement with theory.
中文翻译:

聚电解质与蛋白质的相互作用:量化水的作用
提出了高电荷聚电解质和蛋白质之间复合物形成的自由能 Δ G b的理论模型。这里介绍的模型包括释放的抗衡离子的影响以及复合物形成过程中水分子的吸收或释放。因此,所得的 Δ G b表达式能够描述 Δ G b对温度以及系统中盐浓度的依赖性: 溶液中盐浓度的增加会增加离子的活性和抗衡离子的释放结合变得不太有效。另一方面,盐浓度的增加导致大量水的活性降低。因此,在复合物形成过程中水分子的释放将更加有利,并导致 Δ G b的大小和结合常数的增加。进一步证明,水分子的释放或吸收是在聚电解质与蛋白质形成复合物期间观察到的显着的焓-熵抵消的根源。合成(硫酸化树枝状聚甘油)和天然聚电解质(DNA;肝素)与蛋白质之间形成复合物的实验数据的比较显示与理论完全一致。
更新日期:2021-06-24
中文翻译:

聚电解质与蛋白质的相互作用:量化水的作用
提出了高电荷聚电解质和蛋白质之间复合物形成的自由能 Δ G b的理论模型。这里介绍的模型包括释放的抗衡离子的影响以及复合物形成过程中水分子的吸收或释放。因此,所得的 Δ G b表达式能够描述 Δ G b对温度以及系统中盐浓度的依赖性: 溶液中盐浓度的增加会增加离子的活性和抗衡离子的释放结合变得不太有效。另一方面,盐浓度的增加导致大量水的活性降低。因此,在复合物形成过程中水分子的释放将更加有利,并导致 Δ G b的大小和结合常数的增加。进一步证明,水分子的释放或吸收是在聚电解质与蛋白质形成复合物期间观察到的显着的焓-熵抵消的根源。合成(硫酸化树枝状聚甘油)和天然聚电解质(DNA;肝素)与蛋白质之间形成复合物的实验数据的比较显示与理论完全一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号