Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2021-05-03 , DOI: 10.1016/j.jiec.2021.04.055 Daorong Sun , Zhen Li , Shouqiang Huang , Fengli Yang , Jiawen Chi , Songjian Zhao
|
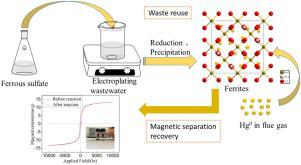
High-quality spinel mixed ferrites (M-Fe) are obtained from the electroplating wastewater, which are then used as adsorbents for the removal of elemental mercury (Hg0) in the flue gas to simultaneously realize the purpose of waste resource utilization and pollution control. In the “ferrite process”, through adjusting the dosages of ferrous sulfate (FeSO4·7H2O), the chromium (Cr) in wastewater can be fully recycled to synthesize the M-Fe adsorbents with good crystal morphology and chemical stability, and they can be easily separated by applying a magnetic field. Hg0 removal experiments indicated that the feeding mass ratio of FeSO4·7H2O: Cr6+ and temperatures had great influence on mercury removal efficiency, and the M-Fe adsorbents with FeSO4·7H2O: Cr6+ mass ratio of 100: 1 (M-Fe (100)) had the highest Hg0 removal performance with nearly 100% at 100℃. In addition, M-Fe (100) presented good sulfur resistance, which remained above 90% Hg0 removal efficiency after SO2 injection, and it can recover activity when stopping SO2. The XPS and desorption dynamics analysis showed mercury existed in the form of physically and chemically adsorbed states. Adsorption kinetic studies manifested that surface active sites were the adsorption rate controlling step, and inner active sites played an important role in mercury adsorption process. Mercury equilibrium analysis indicated mercury amount during adsorption and desorption process was approximately identical, manifesting M-Fe (100) was well recyclable magnetic adsorbent.
中文翻译:

使用从废水中提取的高质量尖晶石混合铁氧体从烟道气中有效去除汞
从电镀废水中提取优质尖晶石混合铁素体(M-Fe),然后作为吸附剂去除烟气中的元素汞(Hg 0),同时实现废物资源化和污染控制的目的. 在“铁素体工艺”中,通过调节硫酸亚铁(FeSO 4 ·7H 2 O)的投加量,可以充分回收废水中的铬(Cr),合成具有良好晶体形貌和化学稳定性的M-Fe吸附剂,它们可以通过施加磁场轻松分离。Hg 0去除实验表明FeSO 4 ·7H 2 O: Cr 6+的进料质量比FeSO 4 ·7H 2 O:Cr 6+质量比为100:1(M-Fe(100))的M-Fe吸附剂对汞的去除效率有很大的影响,对Hg 0 的去除性能最高,接近100℃时为 100%。此外,M-Fe(100)表现出良好的抗硫性,在注入SO 2后仍保持在90%以上的Hg 0去除效率,且在停止SO 2时可恢复活性. XPS 和解吸动力学分析表明汞以物理和化学吸附状态存在。吸附动力学研究表明,表面活性位点是吸附速率的控制步骤,内部活性位点在汞吸附过程中起重要作用。汞平衡分析表明吸附和解吸过程中的汞含量大致相同,表明M-Fe(100)是可很好回收的磁性吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号