当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The ring size of monocyclic ET-1 controls selectivity and signaling efficiency at both endothelin receptor subtypes
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-05-03 , DOI: 10.1002/psc.3325 Philipp Wolf 1 , Annette G Beck-Sickinger 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-05-03 , DOI: 10.1002/psc.3325 Philipp Wolf 1 , Annette G Beck-Sickinger 1
Affiliation
|
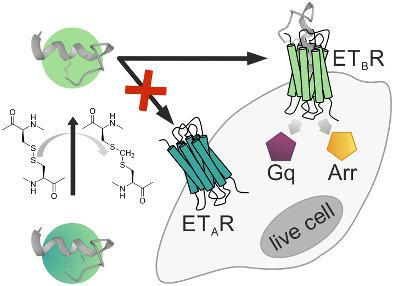
Cardiovascular diseases (CVDs) like hypertension are a major cause for death worldwide. In the cardiovascular tissue, the endothelin system—consisting of the receptor subtypes A (ETAR) and B (ETBR) and the mixed agonist endothelin 1 (ET-1)—is a major key player in the regulation of vascular tone and blood pressure. Tight control of this system is required to maintain homeostasis; otherwise, the endothelin system can cause severe CVDs like pulmonary artery hypertension. The high sequence homology between both receptor subtypes limits the development of novel and selective ligands. Identification of small differences in receptor–ligand interactions and determination of selectivity constraints are crucial to fine-tune ligand properties and subsequent signaling events. Here, we report on novel ET-1 analogs and their detailed pharmacological characterization. We generated simplified ET-1-derived monocyclic peptides to provide an accessible synthesis route. By detailed in vitro characterization, we demonstrated that both G protein signaling and the subsequent arrestin recruitment of activated ETBR remain intact, whereas activation of the ETAR depends on the intramolecular ring size. Increasing of the intramolecular ring structure reduces activity at the ETAR and shifts the peptide toward ETBR selectivity. All ET-1 analogs displayed efficient ETBR-mediated signaling by G protein activation and arrestin 3 recruitment. Our study provides in-depth characterization of the ET-1/ETAR and ET-1/ETBR interactions, which has the potential for future development of endothelin-based drugs for CVD treatment. By identification of Lys9 for selective labeling, novel analogs for peptide-mediated shuttling by ET-1 are proposed.
中文翻译:

单环 ET-1 的环大小控制着两种内皮素受体亚型的选择性和信号效率
高血压等心血管疾病 (CVD) 是全球死亡的主要原因。在心血管组织中,内皮素系统——由受体亚型 A (ET A R) 和 B (ET BR) 和混合激动剂内皮素 1 (ET-1)——是调节血管张力和血压的主要关键参与者。需要对该系统进行严格控制以维持体内平衡;否则,内皮素系统会导致严重的心血管疾病,如肺动脉高压。两种受体亚型之间的高序列同源性限制了新型和选择性配体的开发。识别受体-配体相互作用的微小差异和确定选择性约束对于微调配体特性和随后的信号事件至关重要。在这里,我们报告了新型 ET-1 类似物及其详细的药理学特征。我们生成了简化的 ET-1 衍生的单环肽,以提供一条可行的合成路线。通过详细的体外表征,我们证明了 G 蛋白信号传导和随后激活的 ET B R 的抑制蛋白募集保持完整,而 ET A R 的激活取决于分子内环的大小。分子内环结构的增加降低了在 ET A R处的活性并使肽向 ET B R 选择性转变。所有 ET-1 类似物都通过 G 蛋白激活和抑制蛋白 3 募集显示出有效的 ET B R 介导的信号传导。我们的研究提供了 ET-1/ET A R 和 ET-1/ET B R 相互作用的深入表征,这对于未来开发用于 CVD 治疗的内皮素类药物具有潜力。通过鉴定赖氨酸9用于选择性标记,提出了用于肽介导的 ET-1 穿梭的新型类似物。
更新日期:2021-06-04
中文翻译:

单环 ET-1 的环大小控制着两种内皮素受体亚型的选择性和信号效率
高血压等心血管疾病 (CVD) 是全球死亡的主要原因。在心血管组织中,内皮素系统——由受体亚型 A (ET A R) 和 B (ET BR) 和混合激动剂内皮素 1 (ET-1)——是调节血管张力和血压的主要关键参与者。需要对该系统进行严格控制以维持体内平衡;否则,内皮素系统会导致严重的心血管疾病,如肺动脉高压。两种受体亚型之间的高序列同源性限制了新型和选择性配体的开发。识别受体-配体相互作用的微小差异和确定选择性约束对于微调配体特性和随后的信号事件至关重要。在这里,我们报告了新型 ET-1 类似物及其详细的药理学特征。我们生成了简化的 ET-1 衍生的单环肽,以提供一条可行的合成路线。通过详细的体外表征,我们证明了 G 蛋白信号传导和随后激活的 ET B R 的抑制蛋白募集保持完整,而 ET A R 的激活取决于分子内环的大小。分子内环结构的增加降低了在 ET A R处的活性并使肽向 ET B R 选择性转变。所有 ET-1 类似物都通过 G 蛋白激活和抑制蛋白 3 募集显示出有效的 ET B R 介导的信号传导。我们的研究提供了 ET-1/ET A R 和 ET-1/ET B R 相互作用的深入表征,这对于未来开发用于 CVD 治疗的内皮素类药物具有潜力。通过鉴定赖氨酸9用于选择性标记,提出了用于肽介导的 ET-1 穿梭的新型类似物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号