FlatChem ( IF 5.9 ) Pub Date : 2021-05-03 , DOI: 10.1016/j.flatc.2021.100251 Adil Marjaoui , Mohamed Zanouni , Achraf El Kasmi , Mohammed Jbilou , Mustapha Diani
|
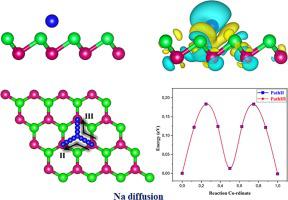
Energy storage technologies in electric vehicles and electronic devices have generated an urgent need for alternative rechargeable batteries such as lithium-ion batteries (LIBs). The cost and limited resources of lithium in the earth's crust may be hindered by the LIBs applications. Due to abundant sodium resources and their similar electrochemical mechanisms, sodium-ion batteries (SIBs) are considered a potential alternative to lithium-ion batteries for large-scale renewable energy storage applications. Herein, based on first-principles density functional theory (DFT), the properties of Na atoms adsorbed on a bismuthene monolayer (ML) have been investigated. Our results exhibited the V site is the optimal adsorption position of Na atoms on ML-Bi with a negative adsorption energy of −1.55 eV and a significant charge transfer. As the adsorption energy of Na increases, the crystal structure of bismuthene tends to be changed significantly and reveals a tendency to form alloys. Also, semiconductor-to-conductor transitions have been observed. Of interest, the diffusion barrier of Na atoms in the NaxBi monolayer system was found to be very low (0.18 eV). This latter would facilitate the insertion/extraction of ions into the electrode material and improves the rate of charge and discharge. These predicted findings can pave the way for experimental studies leading to strong decision-making in the development of battery technology.
中文翻译:

用于电池的铋单层上的钠吸附:第一性原理研究
电动汽车和电子设备中的储能技术迫切需要替代可充电电池,例如锂离子电池(LIB)。LIB的应用可能会阻碍地壳中锂的成本和有限的资源。由于丰富的钠资源及其类似的电化学机制,钠离子电池(SIB)被认为是锂离子电池在大规模可再生能源存储应用中的潜在替代品。在此,基于第一原理密度泛函理论(DFT),研究了吸附在铋单层(ML)上的Na原子的性质。我们的结果表明,V位是Na原子在ML-Bi上的最佳吸附位置,负吸附能为-1.55 eV,电荷转移明显。随着Na的吸附能的增加,铋的晶体结构趋于显着改变,并显示出形成合金的趋势。同样,已经观察到半导体到导体的过渡。有趣的是,Na原子在Na中的扩散势垒X碧单层系统被认为是非常低(0.18电子伏特)。后者将有助于将离子插入/提取到电极材料中并提高充电和放电速率。这些预测的发现可以为进行实验研究铺平道路,从而在电池技术的发展中做出强有力的决策。











































 京公网安备 11010802027423号
京公网安备 11010802027423号