Applied Surface Science ( IF 6.7 ) Pub Date : 2021-05-03 , DOI: 10.1016/j.apsusc.2021.149985 Wenpin Wang , Weijie Wang , Yue Xu , Xiaoxuan Ren , Xien Liu , Zhongcheng Li
|
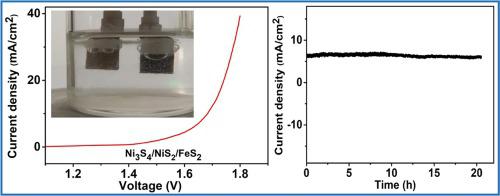
Construction of bi-functional noble metal-free catalysts with low cost and high efficiency is greatly desired for hydrogen and oxygen evolution reactions. It is of vital importance to regulate the surface electron state of pyrite to improve the electrocatalytic performance towards overall water splitting. In this work, we provide a simple one-pot interface-regulated strategy to synthesize Ni3S4/NiS2/FeS2 nanoparticles with exposed interfacial structures between Ni-S and Fe-S, which can then affect the conductivity and activity of pyrite. Notably, Ni3S4/NiS2/FeS2 nanoparticles are highly active in the hydrogen evolution reaction with 197 mV at 10 mA/cm2. Superior oxygen evolution activity is observed for Ni3S4/NiS2/FeS2 nanoparticles with 1.46 V at 10 mA/cm2, outperforming RuO2 with 1.54 V. Impressively, Ni3S4/NiS2/FeS2 nanoparticles could efficiently drive water splitting into hydrogen and oxygen at a voltage of 1.68 V at 10 mA/cm2 in a two-electrode configuration and robust long-term stability was verified by continuous 20 h i-t tests without apparent loss in the current density. The outstanding catalytic activity could be traced back to the presence of interfacial structures between Ni-S and Fe-S. This work highlights that engineering heteroatoms into pyrite that can trigger the generation of interfacial structures, which beneficially accelerates electron transfer during the water splitting process.
中文翻译:

Ni 3 S 4 / NiS 2 / FeS 2纳米粒子的合成及氢氧分解反应
对于氢和氧的放出反应,非常需要低成本和高效率的双功能无贵金属催化剂的构建。调节黄铁矿的表面电子状态以改善对总水分解的电催化性能至关重要。在这项工作中,我们提供了一种简单的一锅法界面调节策略,以合成Ni-S和Fe-S之间具有暴露的界面结构的Ni 3 S 4 / NiS 2 / FeS 2纳米颗粒,然后可以影响其电导率和活性。黄铁矿。值得注意的是,Ni 3 S 4 / NiS 2 / FeS 2纳米粒子在10 mA / cm 2的氢释放反应中具有197 mV的高活性。高级析氧活性观察到的Ni 3小号4 / NIS 2 /的FeS 2个以10毫安/厘米纳米颗粒1.46 V 2,超越的RuO 2与1.54 V.令人印象深刻,镍3小号4 / NIS 2 /的FeS 2层的纳米颗粒能有效地在10 mA / cm 2的电压下以1.68 V的电压驱动将水分解为氢和氧在两个电极的配置中,连续20小时的测试证明了其强大的长期稳定性,而电流密度没有明显损失。出色的催化活性可以追溯到Ni-S和Fe-S之间存在界面结构。这项工作强调了将杂原子工程化为黄铁矿可以触发界面结构的生成,从而有利于加速水分解过程中的电子转移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号