当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Benzyl Alcohol Using Cobalt Oxide Supported Inside and Outside Hollow Carbon Spheres
ChemistryOpen ( IF 2.5 ) Pub Date : 2021-05-02 , DOI: 10.1002/open.202000312 Pumza Mente 1, 2 , Victor Mashindi 2 , Tumelo N Phaahlamohlaka 2 , Thabo N Monyatsi 2 , Roy P Forbes 2 , Neil J Coville 1, 2
ChemistryOpen ( IF 2.5 ) Pub Date : 2021-05-02 , DOI: 10.1002/open.202000312 Pumza Mente 1, 2 , Victor Mashindi 2 , Tumelo N Phaahlamohlaka 2 , Thabo N Monyatsi 2 , Roy P Forbes 2 , Neil J Coville 1, 2
Affiliation
|
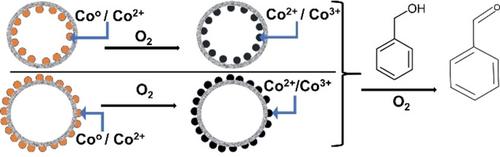
Cobalt oxide nanoparticles (6 nm) supported both inside and outside of hollow carbon spheres (HCSs) were synthesized by using two different polymer templates. The oxidation of benzyl alcohol was used as a model reaction to evaluate the catalysts. PXRD studies indicated that the Co oxidation state varied for the different catalysts due to reduction of the Co by the carbon, and a metal oxidation step prior to the benzyl alcohol oxidation enhanced the catalytic activity. The metal loading influenced the catalytic efficiency, and the activity decreased with increasing metal loading, possibly due to pore filling effects. The catalysts showed similar activity and selectivity (to benzaldehyde) whether placed inside or outside the HCS (63 % selectivity at 50 % conversion). No poisoning was observed due to product build up in the HCS.
中文翻译:

空心碳球内外负载氧化钴氧化苯甲醇
使用两种不同的聚合物模板合成了空心碳球(HCS)内部和外部负载的氧化钴纳米颗粒(6 nm)。使用苯甲醇的氧化作为模型反应来评价催化剂。 PXRD 研究表明,由于 Co 被碳还原,不同催化剂的 Co 氧化态有所不同,并且在苯甲醇氧化之前的金属氧化步骤增强了催化活性。金属负载量影响催化效率,并且活性随着金属负载量的增加而降低,这可能是由于孔填充效应。无论放置在 HCS 内部还是外部,催化剂都表现出相似的活性和选择性(对苯甲醛)(50% 转化率时选择性为 63%)。没有观察到由于 HCS 中产物积聚而导致中毒。
更新日期:2021-06-03
中文翻译:

空心碳球内外负载氧化钴氧化苯甲醇
使用两种不同的聚合物模板合成了空心碳球(HCS)内部和外部负载的氧化钴纳米颗粒(6 nm)。使用苯甲醇的氧化作为模型反应来评价催化剂。 PXRD 研究表明,由于 Co 被碳还原,不同催化剂的 Co 氧化态有所不同,并且在苯甲醇氧化之前的金属氧化步骤增强了催化活性。金属负载量影响催化效率,并且活性随着金属负载量的增加而降低,这可能是由于孔填充效应。无论放置在 HCS 内部还是外部,催化剂都表现出相似的活性和选择性(对苯甲醛)(50% 转化率时选择性为 63%)。没有观察到由于 HCS 中产物积聚而导致中毒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号