当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Experimental Data on Thermodynamic Properties of the Aqueous Solution of N,N-Diethyl-N-methylammonium Bromide and N,N-Diethyl-N-methylammonium Methanesulfonate
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-29 , DOI: 10.1021/acs.jced.1c00111 Marta Królikowska 1, 2 , Katarzyna Grzeszyk 1 , Michał Skonieczny 1 , Marek Królikowski 1, 2 , Maciej Zawadzki 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-29 , DOI: 10.1021/acs.jced.1c00111 Marta Królikowska 1, 2 , Katarzyna Grzeszyk 1 , Michał Skonieczny 1 , Marek Królikowski 1, 2 , Maciej Zawadzki 1
Affiliation
|
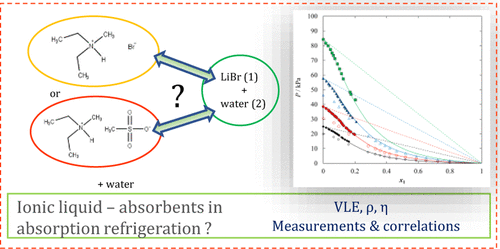
In this work, new experimental data on thermodynamic properties of ionic liquid (IL) aqueous solutions are presented. The (vapor + liquid) phase equilibria, liquid density, and dynamic viscosity of binary mixtures composed of N,N-diethyl-N-methylammonium bromide ([NH,1,2,2][Br]), or N,N-diethyl-N-methylammonium methanesulfonate ([NH,1,2,2][CH3SO3]) and water are presented as functions of temperature and composition. Both ILs were synthesized and specific basic characterization including the NMR spectra and the water content determination was done. The basic thermal properties of the pure IL, including the glass-transition temperature, heat capacity at glass transition, and temperature and enthalpy of (solid + solid) phase transition as well as the temperature and enthalpy of melting were determined using the differential scanning calorimetry (DSC) technique. The isothermal (vapor + liquid) equilibrium (VLE) was measured by the ebulliometric method within a temperature range from T = 338.15 to 368.15 K and pressures up to 85 kPa. Experimental VLE data were successfully correlated using the NRTL equation. The experimental VLE data were tested for thermodynamic consistency using the Van Ness test. The liquid density and the dynamic viscosity were determined as a function of IL’s mole fraction over a wide temperature range. For the correlation of physicochemical properties, empirical equations were applied. From the experimental density data, the excess molar volumes were determined and correlated using the Redlich–Kister-type equation.
中文翻译:

在水溶液中的热力学性质的新实验数据ñ,ñ二乙基ñ -methylammonium溴化物和ñ,ñ二乙基ñ -methylammonium甲基磺酸
在这项工作中,提供了有关离子液体(IL)水溶液热力学性质的新实验数据。由N,N-二乙基-N-甲基溴化铵([ NH,1,2,2 ] [Br])或N,N组成的二元混合物的(蒸气+液相)相平衡,液体密度和动态粘度-二乙基-N-甲基甲磺酸铵([N H,1,2,2 ] [CH 3 SO 3])和水是温度和成分的函数。合成了两种IL,并完成了包括NMR光谱和水含量测定在内的特定基本表征。使用差示扫描量热法测定纯IL的基本热性能,包括玻璃化转变温度,玻璃化转变时的热容量,(固+固)相转变的温度和焓以及熔融温度和熔化焓。 (DSC)技术。等温(蒸气+液体)平衡(VLE)是通过Ebulliometric方法在温度T到T的范围内测量的= 338.15至368.15 K,压力高达85 kPa。使用NRTL方程成功地关联了实验性VLE数据。使用Van Ness检验测试了实验性VLE数据的热力学一致性。在较宽的温度范围内,将液体密度和动态粘度确定为IL摩尔分数的函数。为了理化性质的相关性,使用了经验公式。从实验密度数据中,使用Redlich-Kister型方程式确定并关联了过量的摩尔体积。
更新日期:2021-05-13
中文翻译:

在水溶液中的热力学性质的新实验数据ñ,ñ二乙基ñ -methylammonium溴化物和ñ,ñ二乙基ñ -methylammonium甲基磺酸
在这项工作中,提供了有关离子液体(IL)水溶液热力学性质的新实验数据。由N,N-二乙基-N-甲基溴化铵([ NH,1,2,2 ] [Br])或N,N组成的二元混合物的(蒸气+液相)相平衡,液体密度和动态粘度-二乙基-N-甲基甲磺酸铵([N H,1,2,2 ] [CH 3 SO 3])和水是温度和成分的函数。合成了两种IL,并完成了包括NMR光谱和水含量测定在内的特定基本表征。使用差示扫描量热法测定纯IL的基本热性能,包括玻璃化转变温度,玻璃化转变时的热容量,(固+固)相转变的温度和焓以及熔融温度和熔化焓。 (DSC)技术。等温(蒸气+液体)平衡(VLE)是通过Ebulliometric方法在温度T到T的范围内测量的= 338.15至368.15 K,压力高达85 kPa。使用NRTL方程成功地关联了实验性VLE数据。使用Van Ness检验测试了实验性VLE数据的热力学一致性。在较宽的温度范围内,将液体密度和动态粘度确定为IL摩尔分数的函数。为了理化性质的相关性,使用了经验公式。从实验密度数据中,使用Redlich-Kister型方程式确定并关联了过量的摩尔体积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号