当前位置:
X-MOL 学术
›
Immunol. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Induction of stable human FOXP3+ Tregs by a parasite-derived TGF-β mimic
Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2021-04-30 , DOI: 10.1111/imcb.12475 Laura Cook 1, 2 , Kyle T Reid 2 , Elmeri Häkkinen 2 , Brett Bie 2 , Shigeru Tanaka 3 , Danielle J Smyth 4 , Madeleine PJ White 4 , May Q Wong 1, 2 , Qing Huang 2, 5 , Jana K Gillies 2, 5 , Steven F. Ziegler 3 , Rick M Maizels 4 , Megan K Levings 2, 5, 6
Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2021-04-30 , DOI: 10.1111/imcb.12475 Laura Cook 1, 2 , Kyle T Reid 2 , Elmeri Häkkinen 2 , Brett Bie 2 , Shigeru Tanaka 3 , Danielle J Smyth 4 , Madeleine PJ White 4 , May Q Wong 1, 2 , Qing Huang 2, 5 , Jana K Gillies 2, 5 , Steven F. Ziegler 3 , Rick M Maizels 4 , Megan K Levings 2, 5, 6
Affiliation
|
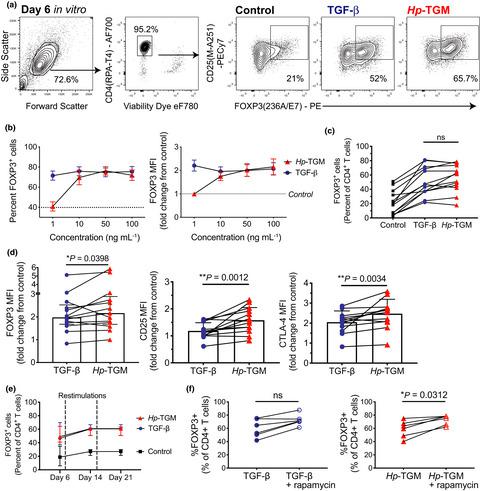
Immune homeostasis in the intestine is tightly controlled by FOXP3+ regulatory T cells (Tregs), defects of which are linked to the development of chronic conditions, such as inflammatory bowel disease (IBD). As a mechanism of immune evasion, several species of intestinal parasites boost Treg activity. The parasite Heligmosomoides polygyrus is known to secrete a molecule (Hp-TGM) that mimics the ability of TGF-β to induce FOXP3 expression in CD4+ T cells. The study aimed to investigate whether Hp-TGM could induce human FOXP3+ Tregs as a potential therapeutic approach for inflammatory diseases. CD4+ T cells from healthy volunteers were expanded in the presence of Hp-TGM or TGF-β. Treg induction was measured by flow cytometric detection of FOXP3 and other Treg markers, such as CD25 and CTLA-4. Epigenetic changes were detected using ChIP-Seq and pyrosequencing of FOXP3. Treg phenotype stability was assessed following inflammatory cytokine challenge and Treg function was evaluated by cellular co-culture suppression assays and cytometric bead arrays for secreted cytokines. Hp-TGM efficiently induced FOXP3 expression (> 60%), in addition to CD25 and CTLA-4, and caused epigenetic modification of the FOXP3 locus to a greater extent than TGF-β. Hp-TGM-induced Tregs had superior suppressive function compared with TGF-β-induced Tregs, and retained their phenotype following exposure to inflammatory cytokines. Furthermore, Hp-TGM induced a Treg-like phenotype in in vivo differentiated Th1 and Th17 cells, indicating its potential to re-program memory cells to enhance immune tolerance. These data indicate Hp-TGM has potential to be used to generate stable human FOXP3+ Tregs to treat IBD and other inflammatory diseases.
中文翻译:

通过寄生虫衍生的 TGF-β 模拟物诱导稳定的人 FOXP3+ Tregs
肠道中的免疫稳态由 FOXP3 +调节性 T 细胞 (Tregs)严格控制,其缺陷与慢性疾病的发展有关,例如炎症性肠病 (IBD)。作为免疫逃避的一种机制,几种肠道寄生虫会促进 Treg 活性。已知寄生虫Heligmosomoides polygyrus会分泌一种分子 ( Hp- TGM),该分子模拟 TGF-β 在 CD4 + T 细胞中诱导 FOXP3 表达的能力。该研究旨在调查Hp- TGM是否可以诱导人类 FOXP3 + Tregs 作为炎症性疾病的潜在治疗方法。CD4 +来自健康志愿者的 T 细胞在Hp -TGM 或 TGF-β存在下扩增。通过流式细胞术检测 FOXP3 和其他 Treg 标记物(如 CD25 和 CTLA-4)来测量 Treg 诱导。使用 ChIP-Seq 和FOXP3焦磷酸测序检测表观遗传变化。在炎症细胞因子攻击后评估 Treg 表型稳定性,并通过细胞共培养抑制测定和细胞计数珠阵列评估分泌细胞因子的 Treg 功能。除了 CD25 和 CTLA-4 外,Hp- TGM 还有效诱导 FOXP3 表达(> 60%),并且比 TGF-β 在更大程度上引起FOXP3基因座的表观遗传修饰。生命值-与 TGF-β 诱导的 Tregs 相比,TGM 诱导的 Tregs 具有更好的抑制功能,并且在暴露于炎性细胞因子后保留了它们的表型。此外,Hp -TGM在体内分化的 Th1 和 Th17 细胞中诱导 Treg 样表型,表明其重新编程记忆细胞以增强免疫耐受性的潜力。这些数据表明Hp- TGM 有可能用于产生稳定的人类 FOXP3 + Tregs 以治疗 IBD 和其他炎症性疾病。
更新日期:2021-04-30
中文翻译:

通过寄生虫衍生的 TGF-β 模拟物诱导稳定的人 FOXP3+ Tregs
肠道中的免疫稳态由 FOXP3 +调节性 T 细胞 (Tregs)严格控制,其缺陷与慢性疾病的发展有关,例如炎症性肠病 (IBD)。作为免疫逃避的一种机制,几种肠道寄生虫会促进 Treg 活性。已知寄生虫Heligmosomoides polygyrus会分泌一种分子 ( Hp- TGM),该分子模拟 TGF-β 在 CD4 + T 细胞中诱导 FOXP3 表达的能力。该研究旨在调查Hp- TGM是否可以诱导人类 FOXP3 + Tregs 作为炎症性疾病的潜在治疗方法。CD4 +来自健康志愿者的 T 细胞在Hp -TGM 或 TGF-β存在下扩增。通过流式细胞术检测 FOXP3 和其他 Treg 标记物(如 CD25 和 CTLA-4)来测量 Treg 诱导。使用 ChIP-Seq 和FOXP3焦磷酸测序检测表观遗传变化。在炎症细胞因子攻击后评估 Treg 表型稳定性,并通过细胞共培养抑制测定和细胞计数珠阵列评估分泌细胞因子的 Treg 功能。除了 CD25 和 CTLA-4 外,Hp- TGM 还有效诱导 FOXP3 表达(> 60%),并且比 TGF-β 在更大程度上引起FOXP3基因座的表观遗传修饰。生命值-与 TGF-β 诱导的 Tregs 相比,TGM 诱导的 Tregs 具有更好的抑制功能,并且在暴露于炎性细胞因子后保留了它们的表型。此外,Hp -TGM在体内分化的 Th1 和 Th17 细胞中诱导 Treg 样表型,表明其重新编程记忆细胞以增强免疫耐受性的潜力。这些数据表明Hp- TGM 有可能用于产生稳定的人类 FOXP3 + Tregs 以治疗 IBD 和其他炎症性疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号