Frontiers of Chemical Science and Engineering ( IF 4.3 ) Pub Date : 2021-04-29 , DOI: 10.1007/s11705-021-2047-9 Iulian Patraşcu , Costin S. Bîldea , Anton A. Kiss
|
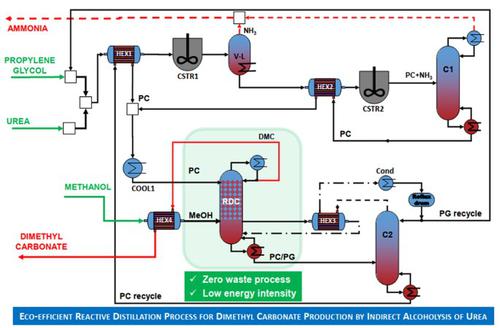
Dimethyl carbonate is an eco-friendly essential chemical that can be sustainably produced from CO2, which is available from carbon capture activities or can even be captured from the air. The rapid increase in dimethyl carbonate demand is driven by the fast growth of polycarbonates, solvent, pharmaceutical, and lithium-ion battery industries. Dimethyl carbonate can be produced from CO2 through various chemical pathways, but the most convenient route reported is the indirect alcoholysis of urea. Previous research used techniques such as heat integration and reactive distillation to reduce the energy use and costs, but the use of an excess of methanol in the trans-esterification step led to an energy intensive extractive distillation required to break the dimethyl carbonate-methanol azeotrope. This work shows that the production of dimethyl carbonate by indirect alcoholysis of urea can be improved by using an excess of propylene carbonate (instead of an excess of methanol), a neat feat that we showed it requires only 2.64 kW·h·kg−1 dimethyl carbonate in a reaction-separation-recycle process, and a reactive distillation column that effectively replaces two conventional distillation columns and the reactor for dimethyl carbonate synthesis. Therefore, less equipment is required, the methanol-dimethyl carbonate azeotrope does not need to be recycled, and the overall savings are higher. Moreover, we propose the use of a reactive distillation column in a heat integrated process to obtain high purity dimethyl carbonate (> 99.8 wt-%). The energy requirement is reduced by heat integration to just 1.25 kW·h·kg−1 dimethyl carbonate, which is about 52% lower than the reaction-separation-recycle process. To benefit from the energy savings, the dynamics and control of the process are provided for ±10% changes in the nominal rate of 32 ktpy dimethyl carbonate, and for uncertainties in reaction kinetics.
中文翻译:

尿素间接醇解生产碳酸二甲酯的新型生态高效反应蒸馏工艺
碳酸二甲酯是一种生态友好型必需化学物质,可以由CO 2可持续生产,而CO 2可通过碳捕获活动获得,甚至可以从空气中捕获。聚碳酸酯,溶剂,制药和锂离子电池行业的快速增长推动了碳酸二甲酯需求的快速增长。碳酸二甲酯可以由CO 2产生通过各种化学途径,但最便捷的途径是尿素的间接醇解。先前的研究使用了诸如热集成和反应蒸馏之类的技术来减少能源使用量和成本,但是在酯交换步骤中使用过量的甲醇导致了需要消耗大量能量的萃取蒸馏来破坏碳酸二甲酯-甲醇共沸物。这项工作表明,通过使用过量的碳酸亚丙酯(而不是过量的甲醇),可以通过尿素的间接醇解来提高碳酸二甲酯的产量,这一整洁的壮举表明我们仅需2.64 kW·h·kg -1在反应-分离-循环过程中使用碳酸二甲酯,以及可有效替代两个常规蒸馏塔和碳酸二甲酯合成反应器的反应性蒸馏塔。因此,需要的设备更少,甲醇-碳酸二甲酯共沸物无需回收,总体节省额更高。此外,我们建议在热集成过程中使用反应蒸馏塔,以获得高纯度碳酸二甲酯(> 99.8 wt%)。通过热集成将能量需求降低到仅1.25 kW·h·kg -1碳酸二甲酯,比反应-分离-循环过程低约52%。为了从节能中受益,为32 ktpy碳酸二甲酯的标称速率提供了±10%的变化,并为反应动力学的不确定性提供了过程的动力学和控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号