Journal of Rare Earths ( IF 5.2 ) Pub Date : 2021-04-29 , DOI: 10.1016/j.jre.2021.04.011 Hongyuan Zhang , Longsheng Zhao , Xudong Zheng , Depeng Liu , Zongyu Feng , Yongqi Zhang , Xiaowei Huang
|
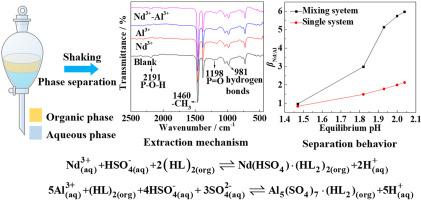
In order to clarify the solvent extraction and separation behaviors of rare earths and impurity of Al during the extraction and enrichment of low-concentration leach solution of ion-adsorption rare earth ore, the extraction mechanism and separation behaviors of Nd3+ and Al3+ in the Nd2(SO4)3–Al2(SO4)3 mixed solution using P507 were studied in this work. The extraction of Nd3+ and Al3+ follows the cation exchange mechanism. With the increase of the equilibrium pH, βNd/Al in the extraction of the Nd2(SO4)3–Al2(SO4)3 mixed solution using P507 is always higher than that in the extraction of single Nd2(SO4)3 and Al2(SO4)3 solutions. It can be attributed to the fact that the extraction of Nd3+ using P507 is much faster than that of Al3+, and Al3+ is more prone to be hydrolyzed at lower pH. βNd/Al in the extraction of the Nd2(SO4)3–Al2(SO4)3 mixed solution decreases gradually with the increase of mixing time within the equilibrium pH range of 1.5–1.9. The extraction of Nd3+ using P507 is much faster than that of Al3+, but the stability of Al3+-loaded organic phase is better than that of Nd3+-loaded organic phase, thus Nd3+ in the Nd3+-loaded organic phase is gradually replaced by Al3+ in the aqueous phase with the increase of mixing time.
中文翻译:

P507-H2SO4体系中低浓度Nd3+和Al3+的萃取机理及分离行为
为阐明离子吸附稀土矿低浓度浸出液萃取富集过程中稀土和杂质Al的溶剂萃取分离行为,Nd 3+和Al 3+的萃取机理及分离行为本工作研究了在 Nd 2 (SO 4 ) 3 -Al 2 (SO 4 ) 3混合溶液中使用 P507。Nd 3+和Al 3+的萃取遵循阳离子交换机制。随着平衡 pH 值的增加,β Nd/Al在 Nd 2 (SO 4) 3 –Al 2 (SO 4 ) 3混合溶液使用P507萃取Nd 2 (SO 4 ) 3和Al 2 (SO 4 ) 3单一溶液时的萃取率始终高于单一溶液。这可以归因于P507对Nd 3+的提取比Al 3+快得多,并且Al 3+在较低pH下更容易水解。β Nd/Al萃取 Nd 2 (SO 4 ) 3 –Al 2 (SO 4 )3混合溶液在1.5-1.9的平衡pH范围内随着混合时间的增加而逐渐减少。P507对Nd 3+的萃取速度远快于Al 3+ ,但负载Al 3+的有机相的稳定性优于负载Nd 3+的有机相,因此Nd 3中的Nd 3+随着混合时间的增加,负载+的有机相逐渐被水相中的Al 3+取代。











































 京公网安备 11010802027423号
京公网安备 11010802027423号