Geochimica et Cosmochimica Acta ( IF 4.5 ) Pub Date : 2021-04-29 , DOI: 10.1016/j.gca.2021.04.027 Wenshuai Li , Xiao-Ming Liu , Yan Hu , Fang-Zhen Teng , Yongfeng Hu
|
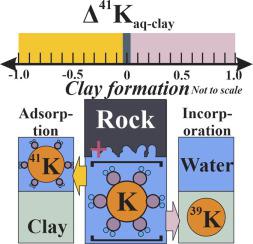
Clay adsorption is a critical process responsible for the mobilization and cycling of potassium (K) on Earth’s surface. Recent studies emphasized the potential of using stable K isotopes (δ41K) to understand chemical weathering. However, the direction, degree, and mechanism of K isotopic fractionation linked to clay K uptake during chemical weathering remain poorly constrained. This work investigated the mechanism of K adsorption on clays (kaolinite and smectite) and the isotopic fractionation in three experimental sets with K-containing solutions. The time-series experiments revealed that the adsorption and isotope equilibria were attained after less than 12-hour reaction. Potassium adsorption rate slowed down and its isotopic fractionation approached the steady-state during 15-day reaction. The pH-dependent experiments demonstrated that the percentage of clay K adsorption and the isotopic composition of adsorbed K (and aqueous K) display negative linear correlations. Net isotopic fractionation between adsorbed and aqueous phases (Δ41Kad-aq) remained near-constant (0.6–0.8‰), regardless of variations in pH ranging from 4 to 10. The concentration-control experiments demonstrated that the percentage of K adsorption decreased with increasing KCl concentrations from 0.005 to 20 mM. The δ41K values of aqueous K reached the minimum of −0.53‰ after 92.7% K adsorbed (initial KCl of 0.005 mM). Potassium adsorption was substantially suppressed as ionic strength (fixed by Na+) increased from 0.001 to 0.5 M without apparent Δ41Kad-aq variations. The K K-edge XANES demonstrated that primary K incorporated in clay lattice and surface KCl derived from sorbed K+ and Cl− synchro-dehydration can be identified after drying of clays. These features indicate that adsorbed K+ was bounded onto clays as outer-sphere complexes, which can be replaced with excess Na+ at high ionic strength. Based on experimental results, we cannot distinguish specific mineralogy regulation on K isotopic fractionation. In sum, isotopically heavy K is preferentially sorbed on clay minerals. The results confirm an equilibrium fractionation path independent of reaction time, pH, ionic strength, and initial KCl concentration. Observed K isotopic fractionation is best fitted by an equilibrium isotopic fractionation law with a fractionation factor αad-aq of 1.00075. We highlight the opposite direction of K isotopic fractionation in clay adsorption and structural incorporation during chemical weathering, and their comparative contributions should be considered for future field investigations.
中文翻译:

粘土吸附过程中钾同位素分馏
粘土吸附是负责钾离子在地球表面上的移动和循环的关键过程。最近的研究强调了使用稳定同位素ķ(δ潜力41 K)了解化学风化。但是,化学风化过程中与粘土K吸收有关的K同位素分馏的方向,程度和机理仍然受限制。 这项工作在含钾溶液的三个实验装置中研究了钾在粘土(高岭石和蒙脱石)上的吸附机理以及同位素分馏。时间序列实验表明,达到了吸附和同位素平衡 少于12小时的反应后。在15天的反应中,钾的吸收速率减慢,其同位素分馏接近稳态。pH依赖的实验表明,粘土K的吸附百分比和吸附的K(和含水K)的同位素组成显示出负线性相关性。吸附和水相(Δ之间净同位素分馏41 ķ广告-AQ)保持在接近恒定的(0.6-0.8‰),与在pH范围从4到10的变化的浓度控制的实验证实,钾的吸附的百分比随着KCl浓度从0.005增至20 mM而降低。该δ 41吸附92.7%的K后(初始KCl为0.005 mM),K水溶液的K值达到-0.53‰的最小值。钾吸附基本上被抑制为离子强度(被Na固定+)为0.001〜0.5M的增加,但没有明显Δ 41 ķ广告水溶液变化。在K ķ _edge时XANES证明在粘土晶格和表面的KCl并入初级ķ从吸附ķ衍生+和Cl -同步-脱水可粘土干燥后进行鉴定。这些特征表明,吸附的K +以外层络合物的形式结合在粘土上,可以被过量的Na +代替在高离子强度下 根据实验结果,我们无法区分K同位素分馏的特定矿物学调控。总之,同位素重的钾优先吸附在粘土矿物上。结果证实了与反应时间,pH,离子强度和初始KCl浓度无关的平衡分馏路径。观察ķ同位素分馏最好是通过用分级因素的平衡同位素分馏法拟合α广告-AQ的1.00075。我们突出了化学风化过程中粘土吸附和结构结合中钾同位素分馏的相反方向,并应考虑它们的比较作用,以供将来进行现场研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号