当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and Thermodynamic Modeling of Lamotrigine Solubility in the Presence of Some Choline-Based Ionic Liquids
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-28 , DOI: 10.1021/acs.jced.1c00073 Hemayat Shekaari 1 , Masumeh Mokhtarpour 1 , Parviz Nesari 1 , Mohammad Khorsandi 1 , Mohammad Reza Behboudi 1 , Behrang Golmohammadi 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-28 , DOI: 10.1021/acs.jced.1c00073 Hemayat Shekaari 1 , Masumeh Mokhtarpour 1 , Parviz Nesari 1 , Mohammad Khorsandi 1 , Mohammad Reza Behboudi 1 , Behrang Golmohammadi 1
Affiliation
|
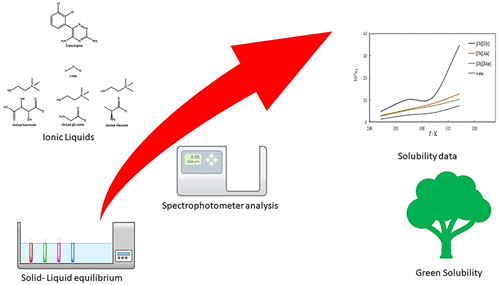
Choline chloride-based ionic liquids (ILs) have recently received a great deal of attention in many fields of science. In this regard, the mole fraction solubility data of lamotrigine (LTG) in the presence of three choline-based ILs were measured from 298.15 to 313.15 K. The LTG is known as an antiepileptic anticonvulsant or antiepileptic drug. This drug has few side effects in comparison to other drugs, and it has a slightly delayed onset of action because of the poor aqueous solubility of LTG (0.187 g/L at 298.15 K). The used ILs (choline glycinate [Ch][Gly], choline alaninate [Ch][Ala], and choline bitartrate [Ch][Bitar]) increased the drug aqueous solubility. Based on the results of solubility studies, it was clear that [Ch][Gly] has a more pronounced effect on LTG solubility enhancement as compared to the two other ILs. Also, in binary solvent systems (ILs and water), the solubility values increased with the rise of temperature. Analysis of the data showed that the solubility behavior was influenced by some properties, including the polarity, hydrogen bonds, cohesive energy density, and molecular structures. The measured data were fitted to some applicable models. The performance of the used models according to %ARD is e-NRTL (0.66%) > Wilson (4.04%) > Jouyban–Acree (5.59%) > Yalkowsky (5.73%) > Apelblat (5.78%). Additionally, the apparent thermodynamic properties of the dissolution process, including the Gibbs energy, enthalpy, and entropy, were calculated using the van’t Hoff and Gibbs equations. The results showed that the dissolution process is an endothermic process and enthalpy-driven in all applied cosolvents. The solubility data for LTG in these neoteric green solvents can be widely used in the pharmaceutical sciences.
中文翻译:

胆碱基离子液体存在下拉莫三嗪溶解度的测量和热力学建模
基于氯化胆碱的离子液体(ILs)最近在许多科学领域受到了广泛关注。在这方面,在三种基于胆碱的ILs的存在下,拉莫三嗪(LTG)的摩尔分数溶解度数据的测量值为298.15至313.15K。LTG被称为抗癫痫药,抗惊厥药或抗癫痫药。与其他药物相比,该药物几乎没有副作用,并且由于LTG的水溶性差(在298.15 K时为0.187 g / L),其起效略有延迟。使用的IL(胆碱甘氨酸盐[Ch] [Gly],胆碱丙氨酸盐[Ch] [Ala]和胆碱酒石酸氢盐[Ch] [Bitar])增加了药物的水溶性。根据溶解度研究的结果,很显然[Ch] [Gly]与其他两种ILs相比,对LTG溶解度的增强作用更为明显。还,在二元溶剂系统(IL和水)中,溶解度值随温度的升高而增加。数据分析表明,溶解度受某些性质的影响,包括极性,氢键,内聚能密度和分子结构。测得的数据适合一些适用的模型。所用模型的性能(按百分比计)ARD为e-NRTL(0.66%)> Wilson(4.04%)> Jouyban–Acree(5.59%)> Yalkowsky(5.73%)> Apelblat(5.78%)。另外,使用van't Hoff和Gibbs方程计算了溶解过程的表观热力学性质,包括吉布斯能量,焓和熵。结果表明,在所有应用的助溶剂中,溶解过程都是一个吸热过程,并且受焓驱动。LTG在这些新型绿色溶剂中的溶解度数据可以在制药科学中广泛使用。
更新日期:2021-05-13
中文翻译:

胆碱基离子液体存在下拉莫三嗪溶解度的测量和热力学建模
基于氯化胆碱的离子液体(ILs)最近在许多科学领域受到了广泛关注。在这方面,在三种基于胆碱的ILs的存在下,拉莫三嗪(LTG)的摩尔分数溶解度数据的测量值为298.15至313.15K。LTG被称为抗癫痫药,抗惊厥药或抗癫痫药。与其他药物相比,该药物几乎没有副作用,并且由于LTG的水溶性差(在298.15 K时为0.187 g / L),其起效略有延迟。使用的IL(胆碱甘氨酸盐[Ch] [Gly],胆碱丙氨酸盐[Ch] [Ala]和胆碱酒石酸氢盐[Ch] [Bitar])增加了药物的水溶性。根据溶解度研究的结果,很显然[Ch] [Gly]与其他两种ILs相比,对LTG溶解度的增强作用更为明显。还,在二元溶剂系统(IL和水)中,溶解度值随温度的升高而增加。数据分析表明,溶解度受某些性质的影响,包括极性,氢键,内聚能密度和分子结构。测得的数据适合一些适用的模型。所用模型的性能(按百分比计)ARD为e-NRTL(0.66%)> Wilson(4.04%)> Jouyban–Acree(5.59%)> Yalkowsky(5.73%)> Apelblat(5.78%)。另外,使用van't Hoff和Gibbs方程计算了溶解过程的表观热力学性质,包括吉布斯能量,焓和熵。结果表明,在所有应用的助溶剂中,溶解过程都是一个吸热过程,并且受焓驱动。LTG在这些新型绿色溶剂中的溶解度数据可以在制药科学中广泛使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号