当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-Term 13C Uptake by 12C-Enriched Calcite
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-04-28 , DOI: 10.1021/acsearthspacechem.0c00122 Antoine Géhin 1 , Benjamin Gilbert 2 , Sudipta Chakraborty 1, 3 , Andrew G. Stack 4 , Lawrence F. Allard 5 , Jean-Charles Robinet 6 , Laurent Charlet 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-04-28 , DOI: 10.1021/acsearthspacechem.0c00122 Antoine Géhin 1 , Benjamin Gilbert 2 , Sudipta Chakraborty 1, 3 , Andrew G. Stack 4 , Lawrence F. Allard 5 , Jean-Charles Robinet 6 , Laurent Charlet 1
Affiliation
|
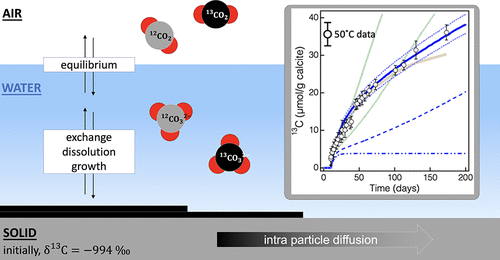
Knowledge of the exchange of carbon isotopes between dissolved inorganic carbon and calcite minerals is of long-standing importance for the interpretation of sedimentary paleoclimate records and 14C transport in the geosphere. To assess the mechanism and rates of carbon isotope exchange, we equilibrated 12C-pure synthetic calcite particles in water, first in a glovebox and then in contact with atmospheric PCO2 and 13C/12C ratio, at two different temperatures. Cavity ring-down infrared spectroscopy δ13C measurements of the solid revealed sustained 13C incorporation for over a period of 500 days (21 °C) and 125 days (50 °C). We developed a quantitative model for recrystallization and isotope exchange, assuming that the interfacial free energy provides a thermodynamic driving force for the growth of larger particles at the expense of smaller ones. However, this Ostwald ripening model did not reproduce the kinetics of 13C uptake and required greater coarsening than observed. Rather, the data were best explained by a mechanism involving surface exchange and solid-phase diffusion of 13C into the particles with an inferred effective diffusion constant at 21 °C of about 10–25 m2/s. Although this work cannot rule out the possibility that structural or chemical aspects of the synthetic particles enabled faster 13C uptake than could be observed in natural systems, this study adds to the body of the recent work, suggesting that fast exchange processes are possible, likely through grain boundaries and other defects.
中文翻译:

富含12 C的方解石的长期13 C吸收
溶解无机碳和方解石矿物之间碳同位素交换的知识对于解释沉积的古气候记录和地球上的14 C迁移具有长期的重要性。为了评估碳同位素交换的机理和速率,我们在两个不同的温度下,首先在手套箱中,然后与大气中的P CO2和13 C / 12 C比接触,平衡了水中的12 C纯合成方解石颗粒。光腔衰荡红外光谱δ 13个固体揭示的Ç测量持续13在500天(21°C)和125天(50°C)的时间内掺入C。我们假设界面自由能以较小的颗粒为代价,为较大的颗粒的生长提供了热力学驱动力,因此我们开发了用于重结晶和同位素交换的定量模型。但是,该奥斯特瓦尔德熟化模型没有重现13 C吸收的动力学,并且需要比观察到的更大的粗化。确切地说,数据是通过一种涉及表面交换和13 C固相扩散到粒子中的机制来最好地解释的,在21°C时推断出的有效扩散常数约为10 – 25 m 2/ s。尽管这项工作不能排除合成颗粒的结构或化学方面比自然系统更快地吸收13 C的可能性,但这项研究增加了最新工作的范围,表明快速交换过程是可能的,可能是通过晶界和其他缺陷。
更新日期:2021-05-20
中文翻译:

富含12 C的方解石的长期13 C吸收
溶解无机碳和方解石矿物之间碳同位素交换的知识对于解释沉积的古气候记录和地球上的14 C迁移具有长期的重要性。为了评估碳同位素交换的机理和速率,我们在两个不同的温度下,首先在手套箱中,然后与大气中的P CO2和13 C / 12 C比接触,平衡了水中的12 C纯合成方解石颗粒。光腔衰荡红外光谱δ 13个固体揭示的Ç测量持续13在500天(21°C)和125天(50°C)的时间内掺入C。我们假设界面自由能以较小的颗粒为代价,为较大的颗粒的生长提供了热力学驱动力,因此我们开发了用于重结晶和同位素交换的定量模型。但是,该奥斯特瓦尔德熟化模型没有重现13 C吸收的动力学,并且需要比观察到的更大的粗化。确切地说,数据是通过一种涉及表面交换和13 C固相扩散到粒子中的机制来最好地解释的,在21°C时推断出的有效扩散常数约为10 – 25 m 2/ s。尽管这项工作不能排除合成颗粒的结构或化学方面比自然系统更快地吸收13 C的可能性,但这项研究增加了最新工作的范围,表明快速交换过程是可能的,可能是通过晶界和其他缺陷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号