Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multigram-scale HPLC enantioseparation as a rescue pathway for circumventing racemization problem during enantioselective synthesis of ethyl 3,4-dihydro-2H-1,4-benzoxazine-2-carboxylate
Chirality ( IF 2.8 ) Pub Date : 2021-04-27 , DOI: 10.1002/chir.23313 Hong‐Ngoc Pham 1, 2 , Axelle Arrault 1 , Nicolas Vanthuyne 3 , Samir Acherar 1
Chirality ( IF 2.8 ) Pub Date : 2021-04-27 , DOI: 10.1002/chir.23313 Hong‐Ngoc Pham 1, 2 , Axelle Arrault 1 , Nicolas Vanthuyne 3 , Samir Acherar 1
Affiliation
|
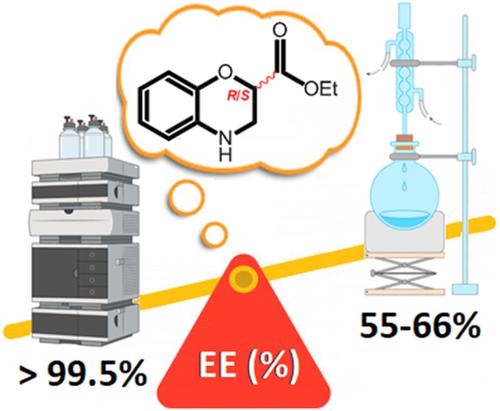
Racemic ethyl 3,4-dihydro-2H-1,4-benzoxazine-2-carboxylate is a key synthon for the design of promising therapeutic drugs. It is mainly synthesized from racemic ethyl 2,3-dibromopropionate and 2-aminophenol in presence of K2CO3 in refluxed acetone. Unfortunately, synthesis of (R)- and (S)-enantiomers using the enantioselective version of this reaction, which should normally be performed with a double SN2 mechanism, is unsuitable due to a racemization process involving the dehydrobromination of enantiopure ethyl 2,3-dibromopropionate into ethyl 2-bromoacrylate. For the first time, the enantioselective version is studied (ee ≈ 55–66%), and the percentage of racemization process has estimated to around 34–46% after determination of the optimal experimental conditions for analytical HPLC enantioseparation of racemate. The influence of the experimental and purification conditions on the racemization rate is also studied. The results indicate that racemization occurs faster at the beginning of the reaction but the initiation of the double SN2 process takes place more faster to limit the racemization rate. The study of the influence of experimental conditions (reaction times, temperature, solvent or type of base, etc.) on the degree of racemization of the (R)- enantiomer is performed and shows that despite changes in the experimental conditions, the synthesis of the (R)- enantiomer is always accompanied by a racemization rate which is difficult in reducing. In parallel, (R)- and (S)-enantiomers are obtained in high enantiopurity (ee > 99.5%) by preparative HPLC enantioseparation of racemate on multigram scale and characterized by optical rotation measurements, ECD and UV spectra.
中文翻译:

在 3,4-二氢-2H-1,4-苯并恶嗪-2-羧酸乙酯的对映选择性合成过程中,多克级 HPLC 对映分离作为规避外消旋问题的拯救途径
外消旋乙基 3,4- dihydro- 2H -1,4-benzoxazine-2-carboxylate 是设计有前景的治疗药物的关键合成子。它主要由外消旋的2,3-二溴丙酸乙酯和2-氨基苯酚在K 2 CO 3存在下在回流的丙酮中合成。不幸的是,使用该反应的对映选择性版本合成 ( R )- 和 ( S )- 对映异构体,这通常应使用双 S N 进行由于涉及对映纯的 2,3-二溴丙酸乙酯脱溴化氢为 2-溴丙烯酸乙酯的外消旋化过程,2 机制不适用。首次研究了对映选择性版本(ee ≈ 55–66%),在确定外消旋物分析 HPLC 对映分离的最佳实验条件后,外消旋过程的百分比估计约为 34–46%。还研究了实验和纯化条件对外消旋化率的影响。结果表明外消旋化在反应开始时发生得更快,但双 S N的引发2 过程发生得更快以限制外消旋化率。进行了实验条件(反应时间、温度、溶剂或碱类型等)对 ( R )- 对映体外消旋化程度的影响的研究,并表明尽管实验条件发生变化,合成( R )-对映体总是伴随着难以降低的外消旋化率。同时,( R )- 和 ( S )- 对映异构体以高对映纯度 (ee > 99.5%) 通过外消旋物的制备型 HPLC 对映分离获得多克规模的外消旋体,并通过旋光度测量、ECD 和 UV 光谱表征。
更新日期:2021-06-08
中文翻译:

在 3,4-二氢-2H-1,4-苯并恶嗪-2-羧酸乙酯的对映选择性合成过程中,多克级 HPLC 对映分离作为规避外消旋问题的拯救途径
外消旋乙基 3,4- dihydro- 2H -1,4-benzoxazine-2-carboxylate 是设计有前景的治疗药物的关键合成子。它主要由外消旋的2,3-二溴丙酸乙酯和2-氨基苯酚在K 2 CO 3存在下在回流的丙酮中合成。不幸的是,使用该反应的对映选择性版本合成 ( R )- 和 ( S )- 对映异构体,这通常应使用双 S N 进行由于涉及对映纯的 2,3-二溴丙酸乙酯脱溴化氢为 2-溴丙烯酸乙酯的外消旋化过程,2 机制不适用。首次研究了对映选择性版本(ee ≈ 55–66%),在确定外消旋物分析 HPLC 对映分离的最佳实验条件后,外消旋过程的百分比估计约为 34–46%。还研究了实验和纯化条件对外消旋化率的影响。结果表明外消旋化在反应开始时发生得更快,但双 S N的引发2 过程发生得更快以限制外消旋化率。进行了实验条件(反应时间、温度、溶剂或碱类型等)对 ( R )- 对映体外消旋化程度的影响的研究,并表明尽管实验条件发生变化,合成( R )-对映体总是伴随着难以降低的外消旋化率。同时,( R )- 和 ( S )- 对映异构体以高对映纯度 (ee > 99.5%) 通过外消旋物的制备型 HPLC 对映分离获得多克规模的外消旋体,并通过旋光度测量、ECD 和 UV 光谱表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号