Cell Stem Cell ( IF 19.8 ) Pub Date : 2021-04-28 , DOI: 10.1016/j.stem.2021.03.022 Ling Huang 1 , Ridhdhi Desai 1 , Daniel N Conrad 2 , Nayara C Leite 3 , Dipikaa Akshinthala 1 , Christine Maria Lim 1 , Raul Gonzalez 4 , Lakshmi B Muthuswamy 1 , Zev Gartner 5 , Senthil K Muthuswamy 1
|
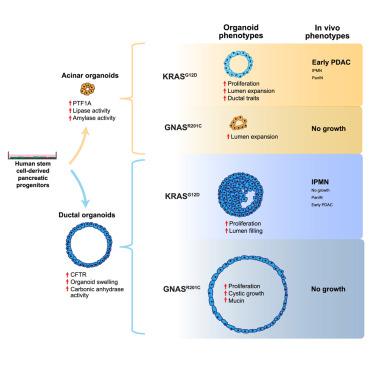
The exocrine pancreas, consisting of ducts and acini, is the site of origin of pancreatitis and pancreatic ductal adenocarcinoma (PDAC). Our understanding of the genesis and progression of human pancreatic diseases, including PDAC, is limited because of challenges in maintaining human acinar and ductal cells in culture. Here we report induction of human pluripotent stem cells toward pancreatic ductal and acinar organoids that recapitulate properties of the neonatal exocrine pancreas. Expression of the PDAC-associated oncogene GNASR201C induces cystic growth more effectively in ductal than acinar organoids, whereas KRASG12D is more effective in modeling cancer in vivo when expressed in acinar compared with ductal organoids. KRASG12D, but not GNASR201C, induces acinar-to-ductal metaplasia-like changes in culture and in vivo. We develop a renewable source of ductal and acinar organoids for modeling exocrine development and diseases and demonstrate lineage tropism and plasticity for oncogene action in the human pancreas.
中文翻译:

人类干细胞衍生的胰腺腺泡和导管类器官的承诺和致癌基因诱导的可塑性
由导管和腺泡组成的外分泌胰腺是胰腺炎和胰腺导管腺癌 (PDAC) 的起源部位。由于在培养中维持人类腺泡和导管细胞方面的挑战,我们对包括 PDAC 在内的人类胰腺疾病的发生和进展的理解是有限的。在这里,我们报告了人类多能干细胞对胰腺导管和腺泡类器官的诱导,这些类器官概括了新生儿外分泌胰腺的特性。PDAC 相关癌基因 GNAS R201C的表达在导管中比在腺泡类器官中更有效地诱导囊性生长,而与导管类器官相比,在腺泡中表达时KRAS G12D在体内建模癌症方面更有效。KRAS G12D,但不是 GNAS R201C,在培养和体内诱导腺泡到导管化生样变化。我们开发了一种可再生的导管和腺泡类器官,用于模拟外分泌发育和疾病,并展示了人类胰腺癌基因作用的谱系趋向性和可塑性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号