当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Data Correlation of Pentaerythritol in Four Binary Solvent Systems at Temperatures from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.jced.1c00080 Shaonan Zhang 1 , Ningning Tian 1 , Shengzhe Jia 1 , Jing Yang 1 , Yu Zhou 1 , Shichao Du 2 , Zhenguo Gao 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.jced.1c00080 Shaonan Zhang 1 , Ningning Tian 1 , Shengzhe Jia 1 , Jing Yang 1 , Yu Zhou 1 , Shichao Du 2 , Zhenguo Gao 1
Affiliation
|
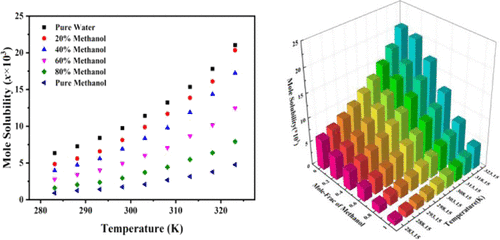
The solubility of pentaerythritol (PE) in four binary solvents (methanol + water, ethanol + water, isopropanol + water, acetonitrile + water) is determined using the gravimetric method at temperatures ranging from 283.15 to 323.15 K under 0.1 MPa. The experimental results demonstrate that the solubility of PE increases with the increase of temperature in all four binary solvent systems. And water has a relatively positive effect on the solubility of PE in all four binary solvents. However, the solubility of PE in ethanol + water and isopropanol + water mixtures first increases with the increase of the ethanol/isopropanol fraction and then decreases when the temperature exceeds 303.15 and 288.15 K, respectively. And the solubility reaches the maximum when the initial mole fractions of ethanol/isopropanol of the binary solvent are 0.2. Besides, experimental solubility is fitted with three thermodynamic models including the modified Apelblat model, λh model, and CNIBS/R-K model, and all of the average relative deviation (ARDs) are less than 4%, indicating good accordance with the experimental results. In addition, among the three thermodynamic models, the CNIBS/R-K model has the best fitting performance, in which all of the ARDs are less than 1.7%.
中文翻译:

季戊四醇在283.15至323.15 K的温度下在四元溶剂体系中的溶解度测量和数据相关性
季戊四醇(PE)在四种二元溶剂(甲醇+水,乙醇+水,异丙醇+水,乙腈+水)中的溶解度使用重量法在0.1 MPa下于283.15至323.15 K范围内测定。实验结果表明,在所有四种二元溶剂体系中,PE的溶解度均随温度的升高而增加。水对PE在所有四种二元溶剂中的溶解度都具有相对积极的影响。但是,PE在乙醇+水和异丙醇+水混合物中的溶解度首先随乙醇/异丙醇馏分的增加而增加,然后在温度分别超过303.15和288.15 K时降低。当二元溶剂的乙醇/异丙醇的初始摩尔分数为0.2时,溶解度达到最大值。除了,h模型和CNIBS / RK模型,所有平均相对偏差(ARDs)均小于4%,表明与实验结果吻合良好。此外,在三种热力学模型中,CNIBS / RK模型具有最佳拟合性能,其中所有ARDs均小于1.7%。
更新日期:2021-05-13
中文翻译:

季戊四醇在283.15至323.15 K的温度下在四元溶剂体系中的溶解度测量和数据相关性
季戊四醇(PE)在四种二元溶剂(甲醇+水,乙醇+水,异丙醇+水,乙腈+水)中的溶解度使用重量法在0.1 MPa下于283.15至323.15 K范围内测定。实验结果表明,在所有四种二元溶剂体系中,PE的溶解度均随温度的升高而增加。水对PE在所有四种二元溶剂中的溶解度都具有相对积极的影响。但是,PE在乙醇+水和异丙醇+水混合物中的溶解度首先随乙醇/异丙醇馏分的增加而增加,然后在温度分别超过303.15和288.15 K时降低。当二元溶剂的乙醇/异丙醇的初始摩尔分数为0.2时,溶解度达到最大值。除了,h模型和CNIBS / RK模型,所有平均相对偏差(ARDs)均小于4%,表明与实验结果吻合良好。此外,在三种热力学模型中,CNIBS / RK模型具有最佳拟合性能,其中所有ARDs均小于1.7%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号