当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Correlation of Solubility of Quizalofop-p-ethyl in Seven Pure Solvents and Three Binary Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.jced.1c00092 Li Yan 1 , Yunhe Bai 1 , Yang Ye 1 , Lianyou Yu 2 , Qi Wang 3 , Chuang Xie 1, 4
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.jced.1c00092 Li Yan 1 , Yunhe Bai 1 , Yang Ye 1 , Lianyou Yu 2 , Qi Wang 3 , Chuang Xie 1, 4
Affiliation
|
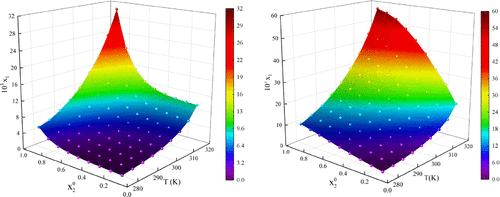
The solubility of quizalofop-p-ethyl (QPE) in seven pure solvents and three binary solvents (n-heptane + ethanol/ethyl acetate/acetone) in the temperature range from 278.15 to 318.15 K was determined by using the gravimetric method. It is found that the solubility of QPE in the seven pure solvents from 278.15 to 303.15 K follows the order: acetone > ethyl acetate > isobutyl alcohol > tert-butanol > ethanol > n-heptane > n-hexane. In each pure solvent, the QPE solubility increases with increasing temperature. In the three binary solvent systems, the QPE solubility increases with increasing temperature and increasing proportion of good solvent, i.e., ethanol/ethyl acetate/acetone. The experimental solubility of QPE in pure solvents was correlated by the modified Apelblat equation, λh model, and NRTL model, among which modified the Apelblat model gave the best accuracy with RMSD and ARD of less than 0.8100 × 10–4 and 10.26%, respectively. The solubility in the binary solvent systems was correlated by the λh model, NRTL model, and CNIBS/R-K equation, which also provided good correlation accuracy with RMSD and ARD less than 1.258 × 10–3 and 6.61%, respectively. Furthermore, the thermodynamic properties including the mixed thermodynamic properties of Gibbs energy change (ΔmixG), molar enthalpy change (ΔmixH), and molar entropy change (ΔmixS) were calculated from the experimental solubility data by using the NRTL model. The negative ΔmixG and positive ΔmixH and ΔmixS indicate that the mixed processes of QPE in the test solvent systems are all endothermic and entropically favorable. The experimental solubility and the models used in this work would be conducive to purifying QPE via crystallization.
中文翻译:

喹喔啉对乙基在七种纯溶剂和三种二元溶剂中的溶解度的测定及相关性
quizalofop-的溶解度p -乙基(QPE)在七个纯溶剂和三个二进制溶剂(Ñ庚烷+乙醇/乙酸乙酯/丙酮)的温度范围内从278.15到318.15 K的通过使用重量法测定。可知QPE的在七个纯溶剂303.15 k中的溶解度从278.15如下顺序:丙酮>乙酸乙酯>异丁醇>叔丁醇>乙醇> Ñ庚烷> Ñ正己烷。在每种纯溶剂中,QPE溶解度随温度升高而增加。在三种二元溶剂系统中,QPE的溶解度随温度的升高和优质溶剂的比例的增加而增加,即,乙醇/乙酸乙酯/丙酮。QPE的在纯溶剂的实验溶解度通过改性Apelblat方程,λ相关ħ模型,和NRTL模型,其中修改了Apelblat模型给出了最佳精度RMSD和小于0.8100×10 ARD -4和10.26%,分别。在二进制的溶剂体系中的溶解度是由λ相关ħ模型,NRTL模型,和CNIBS / RK方程,这也小于1.258×10提供良好的相关性精度,RMSD和ARD -3和6.61%之间。此外,热力学性质包括吉布斯能量变化(Δ的混合热力学性质混合ģ),摩尔焓变化(Δ混合ħ),和摩尔熵变化(Δ混合小号)从实验溶解度数据通过使用NRTL模型计算。负Δ混合G和正Δ混合H和Δ混合S表示测试溶剂系统中QPE的混合过程都是吸热的,并且在熵方面有利。实验工作中的溶解度和模型将有助于通过结晶纯化QPE。
更新日期:2021-05-13
中文翻译:

喹喔啉对乙基在七种纯溶剂和三种二元溶剂中的溶解度的测定及相关性
quizalofop-的溶解度p -乙基(QPE)在七个纯溶剂和三个二进制溶剂(Ñ庚烷+乙醇/乙酸乙酯/丙酮)的温度范围内从278.15到318.15 K的通过使用重量法测定。可知QPE的在七个纯溶剂303.15 k中的溶解度从278.15如下顺序:丙酮>乙酸乙酯>异丁醇>叔丁醇>乙醇> Ñ庚烷> Ñ正己烷。在每种纯溶剂中,QPE溶解度随温度升高而增加。在三种二元溶剂系统中,QPE的溶解度随温度的升高和优质溶剂的比例的增加而增加,即,乙醇/乙酸乙酯/丙酮。QPE的在纯溶剂的实验溶解度通过改性Apelblat方程,λ相关ħ模型,和NRTL模型,其中修改了Apelblat模型给出了最佳精度RMSD和小于0.8100×10 ARD -4和10.26%,分别。在二进制的溶剂体系中的溶解度是由λ相关ħ模型,NRTL模型,和CNIBS / RK方程,这也小于1.258×10提供良好的相关性精度,RMSD和ARD -3和6.61%之间。此外,热力学性质包括吉布斯能量变化(Δ的混合热力学性质混合ģ),摩尔焓变化(Δ混合ħ),和摩尔熵变化(Δ混合小号)从实验溶解度数据通过使用NRTL模型计算。负Δ混合G和正Δ混合H和Δ混合S表示测试溶剂系统中QPE的混合过程都是吸热的,并且在熵方面有利。实验工作中的溶解度和模型将有助于通过结晶纯化QPE。











































 京公网安备 11010802027423号
京公网安备 11010802027423号