Forensic Chemistry ( IF 2.6 ) Pub Date : 2021-04-27 , DOI: 10.1016/j.forc.2021.100340 Briana A. Capistran , Victoria L. McGuffin , Ruth Waddell Smith
|
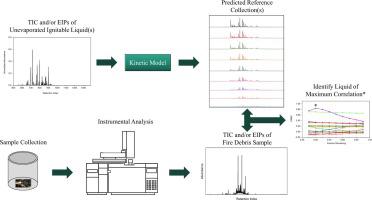
A kinetic model was previously developed in our laboratory to predict evaporation rate constants of compounds in ignitable liquids as a function of gas chromatographic retention index. The model is applied to the chromatogram of an unevaporated liquid and used to predict chromatograms corresponding to any evaporation level of the liquid. In previous work, accuracy in predicting total ion chromatograms (TICs) for gasoline and petroleum distillates was demonstrated. In this work, the model is applied for the first time to predict extracted ion profiles (EIPs) corresponding to different compound classes.
Fifteen ignitable liquids representing five classes (isoparaffinic, naphthenic-paraffinic, aromatic, petroleum distillate, and gasoline) defined in ASTM E1618 were experimentally evaporated to 50–90% (v/v). The kinetic model was applied to predict TICs and EIPs corresponding to the alkane, cycloalkane, aromatic, indane, and polynuclear aromatic compound classes for each evaporated liquid. Strong correlation between experimental and predicted TICs and EIPs indicated the predictive accuracy of the model. Reference collections were then generated containing predicted TICs and EIPs for each liquid corresponding to evaporation levels ranging from 10 to 90%. The reference collections were used to identify the ignitable liquid class in samples of increasing complexity, based on maximum correlation between the experimental and predicted chromatograms. In all cases, the correct liquid class was identified, even in the presence of substrate interferences. Overall, this work demonstrates the potential of a kinetic model to generate TICs and EIPs as a practical tool for identification of ignitable liquids in fire debris samples.
中文翻译:

应用动力学模型预测提取的离子分布,以鉴定蒸发的可燃液体
先前在我们的实验室中开发了一个动力学模型,以预测可燃液体中化合物的蒸发速率常数与气相色谱保留指数的关系。该模型应用于未蒸发液体的色谱图,并用于预测与液体任何蒸发量相对应的色谱图。在以前的工作中,证明了预测汽油和石油馏出物的总离子色谱图(TICs)的准确性。在这项工作中,首次应用该模型来预测对应于不同化合物类别的提取离子图(EIP)。
在ASTM E1618中定义的代表五类(异链烷烃,环烷烃,芳烃,石油馏出物和汽油)的15种可燃液体经实验蒸发至50-90%(v / v)。应用动力学模型预测每种蒸发液体对应于烷烃,环烷烃,芳族化合物,茚满和多核芳族化合物类别的TICs和EIPs。实验和预测的TIC和EIP之间的强相关性表明了模型的预测准确性。然后生成参考集合,其中包含每种液体的预测TIC和EIP,对应于10%至90%的蒸发水平。基于实验色谱图和预测色谱图之间的最大相关性,参考集合用于识别复杂性不断增加的样品中的可燃液体类别。在所有情况下,即使存在底物干扰,也能识别出正确的液体类别。总的来说,这项工作证明了动力学模型产生TICs和EIPs的潜力,将其作为鉴定火屑样品中可燃液体的实用工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号