当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Encapsulation of Boron Nanoparticles Using Reduced Graphene Oxide on their Oxidation Characteristics
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2021-04-27 , DOI: 10.1002/prep.202100004 Hongyi Zhang 1 , Xinyi Du 1 , Gang Li 1, 2
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2021-04-27 , DOI: 10.1002/prep.202100004 Hongyi Zhang 1 , Xinyi Du 1 , Gang Li 1, 2
Affiliation
|
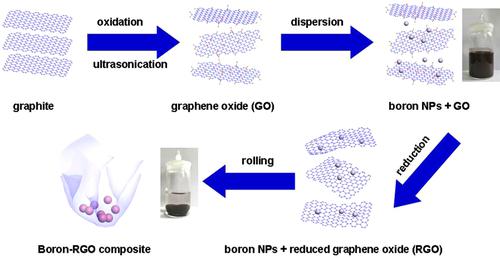
Boron is a well-known high-energy solid. A thick oxide coating on the particle, however, inhibits its ignition and reduces its energy significantly. In this work, graphene nanosheets were used as a protective material for encapsulating boron nanoparticles (NPs) to prevent their oxidation from the air at room temperature. Boron-reduced graphene oxide (RGO) composites were prepared under hydrothermal conditions using graphene oxide (GO) and commercial or washed boron NPs as precursors. Boron-RGO composites were characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), thermogravimetry (TG), and differential scanning calorimetry (DSC). XPS data show that no boron oxide or boric acid was present on the surface of washed boron particles and boron-RGO composites. It seems that the removal of boron oxide or boric acid layer on the commercial boron particle surface and the reduction of graphene oxide occurred simultaneously during hydrothermal treatment. XRD data show that boric acid re-formed on washed boron NPs but not on the boron-RGO composites after these two samples had been exposed to ambient temperature air for four months. This indicates that the anti-oxidation stability of boron against air at room temperature was improved greatly after encapsulation of reduced graphene oxide. The heat of combustion of boron-RGO composites (46.6∼49.3 kJ ⋅ g−1) measured by oxygen bomb calorimetry was higher than that of the commercial boron NPs (43.4 kJ ⋅ g−1). Upon heating, the boron-RGO composite begins oxidizing at a lower temperature than the commercial boron particles. A mechanism was proposed for encapsulation of boron NPs with RGO.
中文翻译:

使用还原氧化石墨烯包封硼纳米颗粒对其氧化特性的影响
硼是一种众所周知的高能固体。然而,颗粒上的厚氧化物涂层会抑制其点火并显着降低其能量。在这项工作中,石墨烯纳米片被用作封装硼纳米颗粒 (NPs) 的保护材料,以防止它们在室温下从空气中氧化。硼还原氧化石墨烯 (RGO) 复合材料是在水热条件下使用氧化石墨烯 (GO) 和商业或洗涤的硼纳米粒子作为前体制备的。硼-RGO 复合材料使用 X 射线衍射 (XRD)、透射电子显微镜 (TEM)、拉曼光谱、X 射线光电子能谱 (XPS)、热重 (TG) 和差示扫描量热法 (DSC) 进行表征。XPS 数据表明,在清洗过的硼颗粒和硼-RGO 复合材料的表面上不存在氧化硼或硼酸。似乎在水热处理期间,商业硼颗粒表面上氧化硼或硼酸层的去除和氧化石墨烯的还原同时发生。XRD 数据表明,在这两个样品暴露于环境温度空气中四个月后,硼酸在清洗过的硼纳米颗粒上重新形成,但在硼-RGO 复合材料上没有重新形成。这表明硼在室温下对空气的抗氧化稳定性在被还原的氧化石墨烯包封后大大提高。硼-RGO 复合材料的燃烧热 (46.6∼49.3 kJ ⋅ g XRD 数据表明,在这两个样品暴露于环境温度空气中四个月后,硼酸在清洗过的硼纳米颗粒上重新形成,但在硼-RGO 复合材料上没有重新形成。这表明硼在室温下对空气的抗氧化稳定性在被还原的氧化石墨烯包封后大大提高。硼-RGO 复合材料的燃烧热 (46.6∼49.3 kJ ⋅ g XRD 数据表明,在这两个样品暴露于环境温度空气中四个月后,硼酸在清洗过的硼纳米颗粒上重新形成,但在硼-RGO 复合材料上没有重新形成。这表明硼在室温下对空气的抗氧化稳定性在被还原的氧化石墨烯包封后大大提高。硼-RGO 复合材料的燃烧热 (46.6∼49.3 kJ ⋅ g-1 ) 通过氧弹量热法测量的高于商业硼纳米粒子 (43.4 kJ ⋅ g -1 )。加热后,硼-RGO 复合材料在比商业硼颗粒更低的温度下开始氧化。提出了一种用 RGO 封装硼纳米颗粒的机制。
更新日期:2021-04-27
中文翻译:

使用还原氧化石墨烯包封硼纳米颗粒对其氧化特性的影响
硼是一种众所周知的高能固体。然而,颗粒上的厚氧化物涂层会抑制其点火并显着降低其能量。在这项工作中,石墨烯纳米片被用作封装硼纳米颗粒 (NPs) 的保护材料,以防止它们在室温下从空气中氧化。硼还原氧化石墨烯 (RGO) 复合材料是在水热条件下使用氧化石墨烯 (GO) 和商业或洗涤的硼纳米粒子作为前体制备的。硼-RGO 复合材料使用 X 射线衍射 (XRD)、透射电子显微镜 (TEM)、拉曼光谱、X 射线光电子能谱 (XPS)、热重 (TG) 和差示扫描量热法 (DSC) 进行表征。XPS 数据表明,在清洗过的硼颗粒和硼-RGO 复合材料的表面上不存在氧化硼或硼酸。似乎在水热处理期间,商业硼颗粒表面上氧化硼或硼酸层的去除和氧化石墨烯的还原同时发生。XRD 数据表明,在这两个样品暴露于环境温度空气中四个月后,硼酸在清洗过的硼纳米颗粒上重新形成,但在硼-RGO 复合材料上没有重新形成。这表明硼在室温下对空气的抗氧化稳定性在被还原的氧化石墨烯包封后大大提高。硼-RGO 复合材料的燃烧热 (46.6∼49.3 kJ ⋅ g XRD 数据表明,在这两个样品暴露于环境温度空气中四个月后,硼酸在清洗过的硼纳米颗粒上重新形成,但在硼-RGO 复合材料上没有重新形成。这表明硼在室温下对空气的抗氧化稳定性在被还原的氧化石墨烯包封后大大提高。硼-RGO 复合材料的燃烧热 (46.6∼49.3 kJ ⋅ g XRD 数据表明,在这两个样品暴露于环境温度空气中四个月后,硼酸在清洗过的硼纳米颗粒上重新形成,但在硼-RGO 复合材料上没有重新形成。这表明硼在室温下对空气的抗氧化稳定性在被还原的氧化石墨烯包封后大大提高。硼-RGO 复合材料的燃烧热 (46.6∼49.3 kJ ⋅ g-1 ) 通过氧弹量热法测量的高于商业硼纳米粒子 (43.4 kJ ⋅ g -1 )。加热后,硼-RGO 复合材料在比商业硼颗粒更低的温度下开始氧化。提出了一种用 RGO 封装硼纳米颗粒的机制。










































 京公网安备 11010802027423号
京公网安备 11010802027423号