Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-04-26 , DOI: 10.1016/j.jmgm.2021.107932 Karina de Paula 1 , Jademilson C Santos 1 , Ana Carolina Mafud 1 , Alessandro S Nascimento 1
|
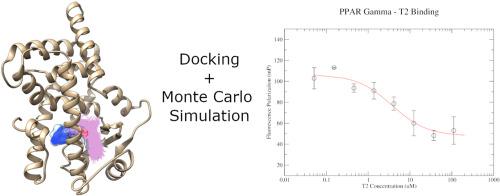
Diabetes is an important chronic disease affecting about 10% of the adult population in the US and over 420 million people worldwide, resulting in 1.6 million deaths every year, according to the World Health Organization. The most common type of the disease, type 2 diabetes, can be pharmacologically managed using oral hypoglycemic agents or thiazolidinediones (TZDs), such as pioglitazone, which act by activating the Peroxisome Proliferated-Activated Receptor γ. Despite their beneficial effects in diabetes treatment, TZDs like rosiglitazone and troglitazone were withdrawn due to safety reasons, creating a void in the pharmacological options for the treatment of this important disease. Here, we explored a structure-based approach in the screening for new chemical probes for a deeper investigation of the effects of PPARγ activation. A class of tetrazole compounds was identified and the compounds named T1, T2 and T3 were purchased and evaluated for their ability to interact with the PPARγ ligand binding domain (LBD). The compounds were binders with micromolar range affinity, as determined by their IC50 values. A Monte Carlo simulation of the compound T2 revealed that the tetrazole ring makes favorable interaction with the polar arm of the receptor binding pocket. Finally, the crystal structure of the PPARγ-LBD-T2 complex was solved at 2.3 Å, confirming the binding mode for this compound. The structure also revealed that, when the helix H12 is mispositioned, an alternative binding conformation is observed for the ligand suggesting an H12-dependent binding conformation for the tetrazole compound.
中文翻译:

四唑作为PPARγ配体的结构和计算研究
根据世界卫生组织的资料,糖尿病是一种重要的慢性疾病,影响到美国约10%的成年人口和全球超过4.2亿人,每年导致160万人死亡。可以使用口服降糖药或噻唑烷二酮(TZD),例如吡格列酮,通过激活过氧化物酶体增殖激活受体γ来进行药理学控制,是最常见的2型糖尿病。尽管它们在糖尿病治疗中具有有益作用,但由于安全性原因,撤回了罗格列酮和曲格列酮等TZD,在治疗这种重要疾病的药理学选择上产生了空白。在这里,我们探索了一种基于结构的方法来筛选新的化学探针,以更深入地研究PPARγ激活的影响。鉴定了一类四唑化合物,并购买了名为T1,T2和T3的化合物,并评估了它们与PPARγ配体结合域(LBD)相互作用的能力。化合物是具有微摩尔范围亲和力的粘合剂,由其IC值确定50个值。化合物T2的Monte Carlo模拟显示,四唑环与受体结合袋的极性臂形成了良好的相互作用。最后,PPARγ-LBD-T2络合物的晶体结构在2.3Å溶解,证实了该化合物的结合模式。该结构还显示,当螺旋H12错位时,观察到配体的替代结合构象,表明四唑化合物具有H12依赖性结合构象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号