Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2021-04-26 , DOI: 10.1016/j.xcrp.2021.100413 Dekun Zhang , Yunrong Chen , Yongning Lai , Xiaoyu Yang
|
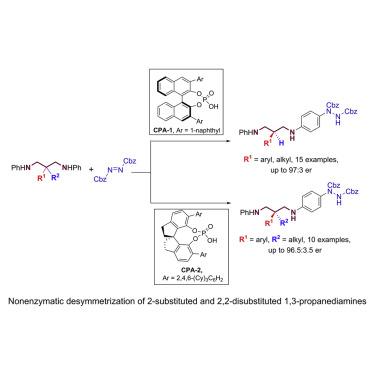
Chiral 1,3-propanediamine moieties are present in a range of bioactive small molecules, chiral catalysts, and ligands. The enantioselective desymmetrization of 2-substituted 1,3-propanediamines is a straightforward and efficient method to give access to these types of enantioenriched compounds. However, the state of the art of this strategy is limited to enzymatic catalysis and 2-monosubstituted substrates. Herein, we report the nonenzymatic desymmetrization of C-2-substituted 1,3-propanediamines through chiral phosphoric-acid-catalyzed asymmetric para-aminations of anilines with azodicarboxylates. Both C-2-monosubstituted and C-2-disubstituted 1,3-propanediamine substrates are compatible with this method, providing chiral 1,3-propanediamines bearing C-2 tertiary and quaternary stereocenters with high enantioselectivities. Preliminary mechanistic studies are performed to shed light on the reaction mechanism and the key asymmetric induction transition state.
中文翻译:

通过苯胺的不对称对氨基氨基化2-取代和2,2-二取代的1,3-丙二胺的对映选择性脱对称
手性的1,3-丙二胺部分存在于一系列生物活性小分子,手性催化剂和配体中。2-取代的1,3-丙二胺的对映选择性去对称化是获得这些类型的对映体富集化合物的直接有效的方法。然而,该策略的技术水平仅限于酶催化和2-单取代的底物。在这里,我们报告通过手性磷酸催化的不对称对位的C-2-取代的1,3-丙二胺的非酶去对称化-苯胺与偶氮二羧酸酯的胺化。C-2-单取代的和C-2-二取代的1,3-丙二胺底物均与该方法兼容,从而提供了具有高对映选择性的带有C-2叔和季立体中心的手性1,3-丙二胺。进行了初步的机理研究,以阐明反应机理和关键的不对称感应转变态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号