当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The structure of POMGNT2 provides new insights into the mechanism to determine the functional O-mannosylation site on α-dystroglycan
Genes to Cells ( IF 1.3 ) Pub Date : 2021-04-23 , DOI: 10.1111/gtc.12853 Rieko Imae 1 , Naoyuki Kuwabara 2 , Hiroshi Manya 1 , Tomohiro Tanaka 3 , Masato Tsuyuguchi 2 , Mamoru Mizuno 3 , Tamao Endo 1 , Ryuichi Kato 2
Genes to Cells ( IF 1.3 ) Pub Date : 2021-04-23 , DOI: 10.1111/gtc.12853 Rieko Imae 1 , Naoyuki Kuwabara 2 , Hiroshi Manya 1 , Tomohiro Tanaka 3 , Masato Tsuyuguchi 2 , Mamoru Mizuno 3 , Tamao Endo 1 , Ryuichi Kato 2
Affiliation
|
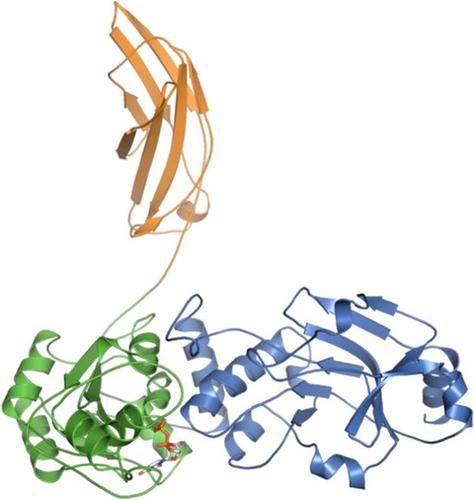
Defects in the O-mannosyl glycan of α-dystroglycan (α-DG) are associated with α-dystroglycanopathy, a group of congenital muscular dystrophies. While α-DG has many O-mannosylation sites, only the specific positions can be modified with the functional O-mannosyl glycan, namely, core M3-type glycan. POMGNT2 is a glycosyltransferase which adds β1,4-linked GlcNAc to the O-mannose (Man) residue to acquire core M3-type glycan. Although it is assumed that POMGNT2 extends the specific O-Man residues around particular amino acid sequences, the details are not well understood. Here, we determined a series of crystal structures of POMGNT2 with and without the acceptor O-mannosyl peptides and identified the critical interactions between POMGNT2 and the acceptor peptide. POMGNT2 has an N-terminal catalytic domain and a C-terminal fibronectin type III (FnIII) domain and forms a dimer. The acceptor peptide is sandwiched between the two protomers. The catalytic domain of one protomer recognizes the O-mannosylation site (TPT motif), and the FnIII domain of the other protomer recognizes the C-terminal region of the peptide. Structure-based mutational studies confirmed that amino acid residues of the catalytic domain interacting with mannose or the TPT motif are essential for POMGNT2 enzymatic activity. In addition, the FnIII domain is also essential for the activity and it interacts with the peptide mainly by hydrophobic interaction. Our study provides the first atomic-resolution insights into specific acceptor recognition by the FnIII domain of POMGNT2. The catalytic mechanism of POMGNT2 is proposed based on the structure.
中文翻译:

POMGNT2的结构为确定α-dystroglycan上功能性O-甘露糖基化位点的机制提供了新的见解
α-dystroglycan (α-DG)的O-甘露糖基聚糖缺陷与 α-dystroglycanopathy(一组先天性肌营养不良症)有关。虽然α-DG有许多O-甘露糖基化位点,但只有特定位置可以用功能性O-甘露糖基聚糖进行修饰,即核心M3型聚糖。POMGNT2 是一种糖基转移酶,它将 β1,4 连接的 GlcNAc 添加到O-甘露糖 (Man) 残基上以获得核心 M3 型聚糖。尽管假设 POMGNT2 扩展了特定氨基酸序列周围的特定O- Man残基,但细节尚不清楚。在这里,我们确定了有和没有受体O的 POMGNT2 的一系列晶体结构-甘露糖肽并确定了 POMGNT2 和受体肽之间的关键相互作用。POMGNT2 具有 N 端催化结构域和 C 端纤连蛋白 III 型 (FnIII) 结构域并形成二聚体。受体肽夹在两个原体之间。一个原体的催化域识别O-甘露糖基化位点(TPT 基序),另一个原体的 FnIII 结构域识别肽的 C 端区域。基于结构的突变研究证实,催化结构域的氨基酸残基与甘露糖或 TPT 基序相互作用对于 POMGNT2 酶活性是必不可少的。此外,FnIII 结构域对于活性也是必不可少的,它主要通过疏水相互作用与肽相互作用。我们的研究提供了对 POMGNT2 的 FnIII 域识别特定受体的第一个原子分辨率见解。基于该结构提出了POMGNT2的催化机理。
更新日期:2021-04-23
中文翻译:

POMGNT2的结构为确定α-dystroglycan上功能性O-甘露糖基化位点的机制提供了新的见解
α-dystroglycan (α-DG)的O-甘露糖基聚糖缺陷与 α-dystroglycanopathy(一组先天性肌营养不良症)有关。虽然α-DG有许多O-甘露糖基化位点,但只有特定位置可以用功能性O-甘露糖基聚糖进行修饰,即核心M3型聚糖。POMGNT2 是一种糖基转移酶,它将 β1,4 连接的 GlcNAc 添加到O-甘露糖 (Man) 残基上以获得核心 M3 型聚糖。尽管假设 POMGNT2 扩展了特定氨基酸序列周围的特定O- Man残基,但细节尚不清楚。在这里,我们确定了有和没有受体O的 POMGNT2 的一系列晶体结构-甘露糖肽并确定了 POMGNT2 和受体肽之间的关键相互作用。POMGNT2 具有 N 端催化结构域和 C 端纤连蛋白 III 型 (FnIII) 结构域并形成二聚体。受体肽夹在两个原体之间。一个原体的催化域识别O-甘露糖基化位点(TPT 基序),另一个原体的 FnIII 结构域识别肽的 C 端区域。基于结构的突变研究证实,催化结构域的氨基酸残基与甘露糖或 TPT 基序相互作用对于 POMGNT2 酶活性是必不可少的。此外,FnIII 结构域对于活性也是必不可少的,它主要通过疏水相互作用与肽相互作用。我们的研究提供了对 POMGNT2 的 FnIII 域识别特定受体的第一个原子分辨率见解。基于该结构提出了POMGNT2的催化机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号