Chemical Geology ( IF 3.6 ) Pub Date : 2021-04-24 , DOI: 10.1016/j.chemgeo.2021.120229 Pooya Paydary , Alexandra E.P. Schellenger , Minerva Teli , Deb P. Jaisi , Annalisa Onnis-Hayden , Philip Larese-Casanova
|
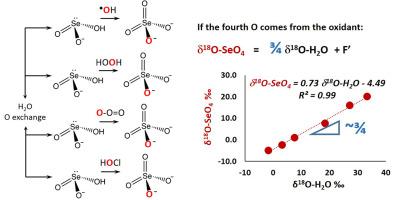
Naturally occurring selenium is usually found in shales, mostly in the form of reduced selenides or elemental selenium metal. In contrast, downstream oxic waters and soils contain the oxidized forms of selenium as the oxyanions selenite (HSeO3−, SeO32−) and selenate (SeO42−). Whereas the oxidation of selenides to selenium oxyanions is possible in the presence of O2, the actual mechanisms of oxidation, and selenate formation in particular, are not fully understood. In this work, reactive oxygen species were evaluated for selenite oxidation within batch reactors at circumneutral pH. Complete selenite oxidation to selenate by ozone (O3) and hypochlorite (OCl−, as a positive control) occurred within minutes and seconds, respectively. Partial oxidation of selenite to selenate by hydrogen peroxide required two weeks reaction at 2 M H2O2. Hydroxyl radicals were generated by photocatalytic decomposition of H2O2 and oxidized selenite completely within six hours. Singlet oxygen, superoxide, and peroxynitrite were not observed to oxidize selenite. By using selenite and H2O with varying δ18O isotopic compositions in oxidation experiments, it was possible to infer the two different sources of O during selenate formation. Selenate inherits three O from selenite and is suggested to acquire the fourth O via O transfer from the oxidants studied here.
中文翻译:

亚硒酸盐化学氧化成硒酸盐:活性氧和O转移途径的评估
天然存在的硒通常存在于页岩中,主要以还原硒化物或元素硒金属的形式存在。与此相反,下游好氧水域和土壤含有硒作为硒氧阴离子的氧化形式(HSeO 3 -,SEO 3 2-)和硒(SEO 4 2-)。尽管在O 2的存在下硒化物可能被氧化成硒氧阴离子,但具体的氧化机理,尤其是硒酸盐的形成尚不完全清楚。在这项工作中,评估了在环境pH值下间歇式反应器中亚硒酸盐氧化的活性氧种类。完整硒氧化硒通过臭氧(O 3)和次氯酸盐(OCL -,作为阳性对照)分别在数分钟和数秒内发生。过氧化氢将亚硒酸盐部分氧化成硒酸盐需要在2 MH 2 O 2下反应两周。H 2 O 2的光催化分解和在6小时内完全氧化的亚硒酸盐产生了羟基自由基。未观察到单线态氧,超氧化物和过氧亚硝酸盐氧化亚硒酸盐。通过使用亚硒酸盐和H 2 O运用变δ 18 ○在氧化实验同位素组成,有可能在形成硒酸盐来推断的O的两个不同的来源。硒酸盐从亚硒酸盐中继承了三个O,建议从此处研究的氧化剂通过O转移获得第四个O。











































 京公网安备 11010802027423号
京公网安备 11010802027423号