Marine Pollution Bulletin ( IF 5.3 ) Pub Date : 2021-04-22 , DOI: 10.1016/j.marpolbul.2021.112390 Reyhane Madadi , Abdolreza Karbassi , Mohsen Saeedi
|
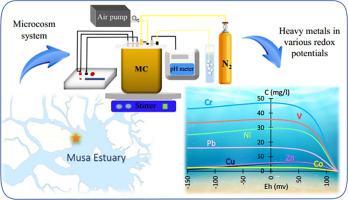
Sediments are capable of adsorbing and desorbing heavy metals (HMs) under various environmental conditions. This study investigated the impact of pre-set redox potential (Eh) on the release dynamics of HMs (Co, Cr, Cu, Ni, Pb, V, and Zn) from sediment in an automated biogeochemical microcosm. The release of Co, Pb, and V under reducing conditions increased that may increase the potential risks in the aquatic environment. This phenomenon could be attributed to the decrease in pH, the reductive dissolution of Fe Mn oxides, and the complex of HMs with dissolved organic carbon (DOC). However, the soluble Cr, Cu, Ni, and Zn decreased at redox potentials as low as −150 mV. Co, Ni, Pb, and Zn were observed in mobile fractions while Cu primarily existed in the residual fraction (indicating lithogenic source). HPI and HEI indexes showed that water quality concerning HMs would become more unsuitable for aquatic life by reducing Eh.
Mn oxides, and the complex of HMs with dissolved organic carbon (DOC). However, the soluble Cr, Cu, Ni, and Zn decreased at redox potentials as low as −150 mV. Co, Ni, Pb, and Zn were observed in mobile fractions while Cu primarily existed in the residual fraction (indicating lithogenic source). HPI and HEI indexes showed that water quality concerning HMs would become more unsuitable for aquatic life by reducing Eh.
中文翻译:

在预设的氧化还原电势下,波斯湾西北部Musa河口沉积物中的重金属释放
沉积物能够在各种环境条件下吸附和解吸重金属(HMs)。这项研究调查了预设的氧化还原电势(Eh)对自动生物地球化学微观世界中沉积物中HMs(Co,Cr,Cu,Ni,Pb,V和Zn)释放动力学的影响。在还原条件下,Co,Pb和V的释放增加,这可能会增加水生环境中的潜在风险。这种现象可能是由于pH降低,铁的还原溶解 Mn氧化物,以及HM与溶解的有机碳(DOC)的配合物。但是,可溶性Cr,Cu,Ni和Zn在低至-150 mV的氧化还原电势下会降低。在流动部分中观察到了Co,Ni,Pb和Zn,而Cu主要存在于残余部分中(表明成岩源)。HPI和HEI指数表明,与HM有关的水质将通过降低Eh而变得更不适合水生生物。
Mn氧化物,以及HM与溶解的有机碳(DOC)的配合物。但是,可溶性Cr,Cu,Ni和Zn在低至-150 mV的氧化还原电势下会降低。在流动部分中观察到了Co,Ni,Pb和Zn,而Cu主要存在于残余部分中(表明成岩源)。HPI和HEI指数表明,与HM有关的水质将通过降低Eh而变得更不适合水生生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号