当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competing Ion Behavior in Direct Electrochemical Selenite Reduction
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-04-22 , DOI: 10.1021/acsestengg.1c00099 Shiqiang Zou 1 , Meagan S. Mauter 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-04-22 , DOI: 10.1021/acsestengg.1c00099 Shiqiang Zou 1 , Meagan S. Mauter 1
Affiliation
|
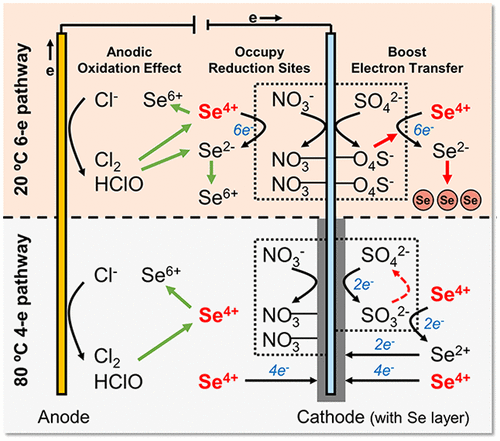
Anthropogenic selenium (Se) released in industrial and agricultural wastewaters presents toxicity challenges for local ecosystems. Se direct electrochemical reduction (SeDER) is an effective and thermodynamically favorable approach for Se(IV) removal, but evaluating the feasibility of SeDER in application requires a comprehensive understanding of system performance in complex water matrices. This study evaluates both the cathodic and anodic competing ion behavior in a SeDER process, including both four- and six-electron Se(IV) reduction pathways. The results suggest that sulfate promotes electrochemical Se(IV) removal efficiency by 11–23%, but nitrate hinders Se(IV) removal (2–11% decrease) by occupying cathodic reaction sites. The anodic competing ions, especially chloride, decrease SeDER performance by generating strong oxidants and disrupting Se(IV) reduction pathways. We also find that four-electron Se(IV) reduction outperforms its six-electron counterpart when treating simulated flue-gas desulfurization wastewater (with 7 g L–1 Cl–), with a lowest effluent Se level of 0.23 mg L–1, a highest removal efficiency of 96.9%, and a threshold deposition capacity of 3.5 g m–2 in a 7-day semicontinuous operation. Electrochemical Se(IV) removal using our prototype batch reactor is not competitive for treating low-Se concentration agricultural wastewaters (up to 24% removal). The results suggest the need for future work to evaluate alternative electrodes and reactor design that reduce water splitting reactions, enhance Faradaic efficiency, and promote mass transfer to the electrode surface.
中文翻译:

直接电化学亚硒酸盐还原中的竞争离子行为
工业和农业废水中释放的人为硒 (Se) 对当地生态系统提出了毒性挑战。Se 直接电化学还原 (SeDER) 是去除 Se(IV) 的有效且热力学有利的方法,但评估 SeDER 在应用中的可行性需要全面了解复杂水基质中的系统性能。本研究评估了 SeDER 过程中的阴极和阳极竞争离子行为,包括四电子和六电子 Se(IV) 还原途径。结果表明,硫酸盐可将电化学 Se(IV) 去除效率提高 11-23%,但硝酸盐通过占据阴极反应位点阻碍 Se(IV) 去除(降低 2-11%)。阳极竞争离子,尤其是氯离子,通过产生强氧化剂和破坏 Se(IV) 还原途径来降低 SeDER 的性能。我们还发现,在处理模拟烟气脱硫废水(7 g L–1 Cl – ),最低出水硒含量为 0.23 mg L –1,最高去除效率为 96.9%,在 7 天半连续操作中的阈值沉积量为 3.5 gm –2。使用我们的原型间歇式反应器进行电化学硒 (IV) 去除在处理低硒浓度农业废水(去除率高达 24%)方面没有竞争力。结果表明,未来需要评估替代电极和反应器设计,以减少水分解反应,提高法拉第效率,并促进向电极表面的传质。
更新日期:2021-06-11
中文翻译:

直接电化学亚硒酸盐还原中的竞争离子行为
工业和农业废水中释放的人为硒 (Se) 对当地生态系统提出了毒性挑战。Se 直接电化学还原 (SeDER) 是去除 Se(IV) 的有效且热力学有利的方法,但评估 SeDER 在应用中的可行性需要全面了解复杂水基质中的系统性能。本研究评估了 SeDER 过程中的阴极和阳极竞争离子行为,包括四电子和六电子 Se(IV) 还原途径。结果表明,硫酸盐可将电化学 Se(IV) 去除效率提高 11-23%,但硝酸盐通过占据阴极反应位点阻碍 Se(IV) 去除(降低 2-11%)。阳极竞争离子,尤其是氯离子,通过产生强氧化剂和破坏 Se(IV) 还原途径来降低 SeDER 的性能。我们还发现,在处理模拟烟气脱硫废水(7 g L–1 Cl – ),最低出水硒含量为 0.23 mg L –1,最高去除效率为 96.9%,在 7 天半连续操作中的阈值沉积量为 3.5 gm –2。使用我们的原型间歇式反应器进行电化学硒 (IV) 去除在处理低硒浓度农业废水(去除率高达 24%)方面没有竞争力。结果表明,未来需要评估替代电极和反应器设计,以减少水分解反应,提高法拉第效率,并促进向电极表面的传质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号