Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2021-04-22 , DOI: 10.1016/j.xcrp.2021.100406 Haiyan Zhang , Jun Huang , Fanke Meng
|
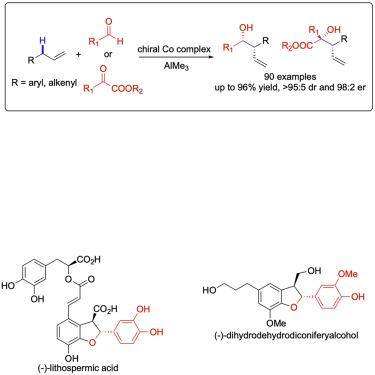
Development of catalytic generation of allyl–metal complexes through allylic C–H cleavage of alkenes without prefunctionalization followed by site- and stereoselective carbon–carbon bond formation is of great importance in organic synthesis, providing a straightforward and step-economical approach to introduce a versatile allyl group into organic molecules. Although significant advances have been achieved in enantioselective transformations of electrophilic allyl–metal complexes and allyl radicals, enantioselective reactions of nucleophilic allyl–metal intermediates furnished through allylic C–H cleavage remain undeveloped. Herein, we identify a multi-tasking chiral catalyst derived from a commercially available phosphine ligand and cobalt salt that precisely controls the chemoselective formation of the allyl–cobalt complex and the site- and stereoselective addition to carbonyls, delivering a broad scope of homoallylic alcohols with high yield and stereoselectivity. This work may establish a platform for the development of enantioselective transformations of nucleophilic organometallic complexes generated from catalytic C–H functionalization.
中文翻译:

通过烯丙基CH官能团将钴催化的非对映和对映选择性烯丙基添加到醛和α-酮酸酯中
在不进行预官能化的情况下,通过烯丙基C–H裂解烯烃进行催化生成烯丙基金属络合物的催化反应,然后形成位点和立体选择性的碳-碳键,这在有机合成中非常重要,它提供了一种简单,经济的方法来引入多用途烯丙基变成有机分子。尽管在亲电烯丙基金属配合物和烯丙基自由基的对映选择性转化方面已取得重大进展,但通过烯丙基C H裂解提供的亲核烯丙基金属中间体的对映选择性反应仍未开发。在此处,我们确定了一种由市售的膦配体和钴盐衍生的多任务手性催化剂,该催化剂可精确地控制烯丙基-钴络合物的化学选择性形成以及羰基的位点和立体选择性加成,从而提供了高收率的多种均丙醇和立体选择性。这项工作可能为开发由催化C–H官能化产生的亲核有机金属配合物的对映选择性转化建立一个平台。









































 京公网安备 11010802027423号
京公网安备 11010802027423号